31 Open Label Extension Study Definition
Oles open label extension study. An open label extension study is one that will lie between a double blind randomized controlled drug trial the parent trial and the request for fda approval.
 Safety Information Eucrisa Crisaborole Rx Treatment
Safety Information Eucrisa Crisaborole Rx Treatment
However a recent medline search for studies between 2000 and 2004 produced only 86 open label studies but over 2000 phase iii studies.

Open label extension study definition. In 2004 the multicentre research ethics committee for wales reviewed three open label extension studies compared with 19 phase iii studies of new drugs a ratio of just over 61. Enrollment into an ole study typically follows enrollment into a randomized blinded well controlled main study. Consumers institutions where these studies are conducted and research ethics committees need to be convinced of the motives as well as the quality of the open label extension study and its execution before supporting such studies.
Consumers institutions where these studies are conducted and research ethics committees need to be convinced of the motives as well as the quality of the open label extension study and its execution before supporting such studies. Open label extension study listed as oles. Open label extension ole studies are common but they do not receive as much attention as traditional phase i through phase iv studies.
Definition of open label extension study open label extension study means with respect to each collaboration program the open label extension trial for such collaboration program described in the pre exercise development plan and budget. Open label extension studies do have a legitimate but limited place in the clinical development of new medicines. Open knowledge foundation network.
In a third phase of the study of 136 patients who had improved with tms therapy in the initial study and in the open label study who were able to resume medication an interim analysis at 4 weeks indicated that the relapse rate was low. It is open label extension study. Open label extension studies do have a legitimate but limited place in the clinical development of new medicines.
Looking for abbreviations of oles.

 Moderate To Severe Chronic Psoriasis Humira 16 Week Efficacy Data
Moderate To Severe Chronic Psoriasis Humira 16 Week Efficacy Data
 Moderate To Severe Chronic Psoriasis Humira 16 Week Efficacy Data
Moderate To Severe Chronic Psoriasis Humira 16 Week Efficacy Data
 Psoriatic Arthritis 3 Year Clinical Trial Results Cosentyx
Psoriatic Arthritis 3 Year Clinical Trial Results Cosentyx
 A Phase 3 Multicenter Open Label 12 Month Extension Safet
A Phase 3 Multicenter Open Label 12 Month Extension Safet
 Safety Information Eucrisa Crisaborole Rx Treatment
Safety Information Eucrisa Crisaborole Rx Treatment
 Efficacy And Safety Of Evolocumab In Reducing Lipids And
Efficacy And Safety Of Evolocumab In Reducing Lipids And
 Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis
Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis
 Long Term Safety And Efficacy Of Bremelanotide For Hypoactiv
Long Term Safety And Efficacy Of Bremelanotide For Hypoactiv
 Nusinersen In Later Onset Spinal Muscular Atrophy Neurology
Nusinersen In Later Onset Spinal Muscular Atrophy Neurology
 Figure 1 From Olmesartan Amlodipine Hydrochlorothiazide In
Figure 1 From Olmesartan Amlodipine Hydrochlorothiazide In
Long Term Efficacy And Safety Of Evolocumab In Patients With
 Siponimod Versus Placebo In Secondary Progressive Multiple
Siponimod Versus Placebo In Secondary Progressive Multiple
 Long Term Safety And Efficacy Of Sarilumab Plus Methotrexate On
Long Term Safety And Efficacy Of Sarilumab Plus Methotrexate On
 Telotristat Ethyl In Carcinoid Syndrome Safety And Efficacy In
Telotristat Ethyl In Carcinoid Syndrome Safety And Efficacy In
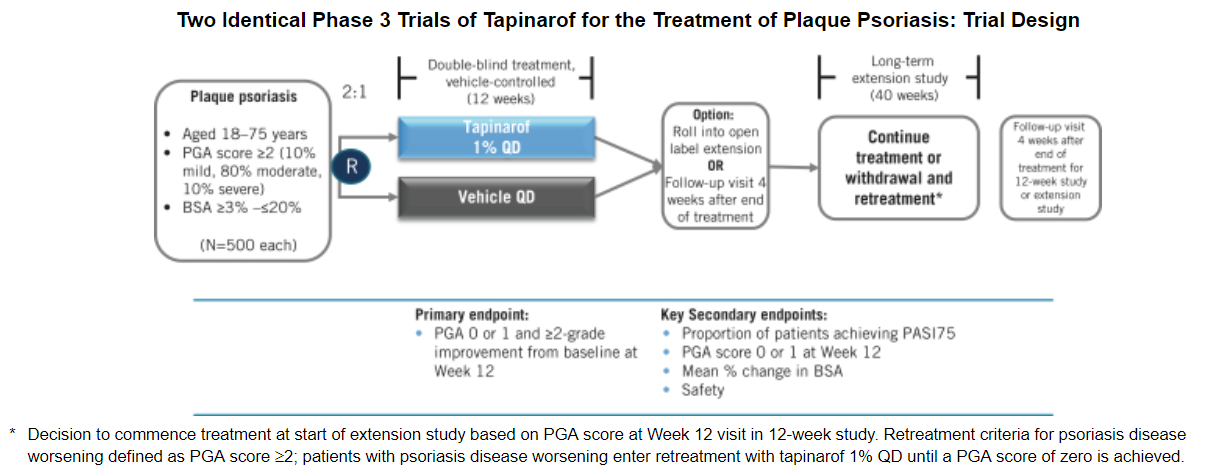 Dermavant Sciences Ipo Buy If The Market Capitalization Is 200
Dermavant Sciences Ipo Buy If The Market Capitalization Is 200
 Open Label Extension Study Of Ranibizumab Image Courtesy Of
Open Label Extension Study Of Ranibizumab Image Courtesy Of
 Cannabidiol In Patients With Lennox Gastaut Syndrome Interim
Cannabidiol In Patients With Lennox Gastaut Syndrome Interim
 Open Label Extension Study Of Ranibizumab Image Courtesy Of
Open Label Extension Study Of Ranibizumab Image Courtesy Of
 Efficacy Of The Pharmacologic Chaperone Migalastat In A Subset Of
Efficacy Of The Pharmacologic Chaperone Migalastat In A Subset Of
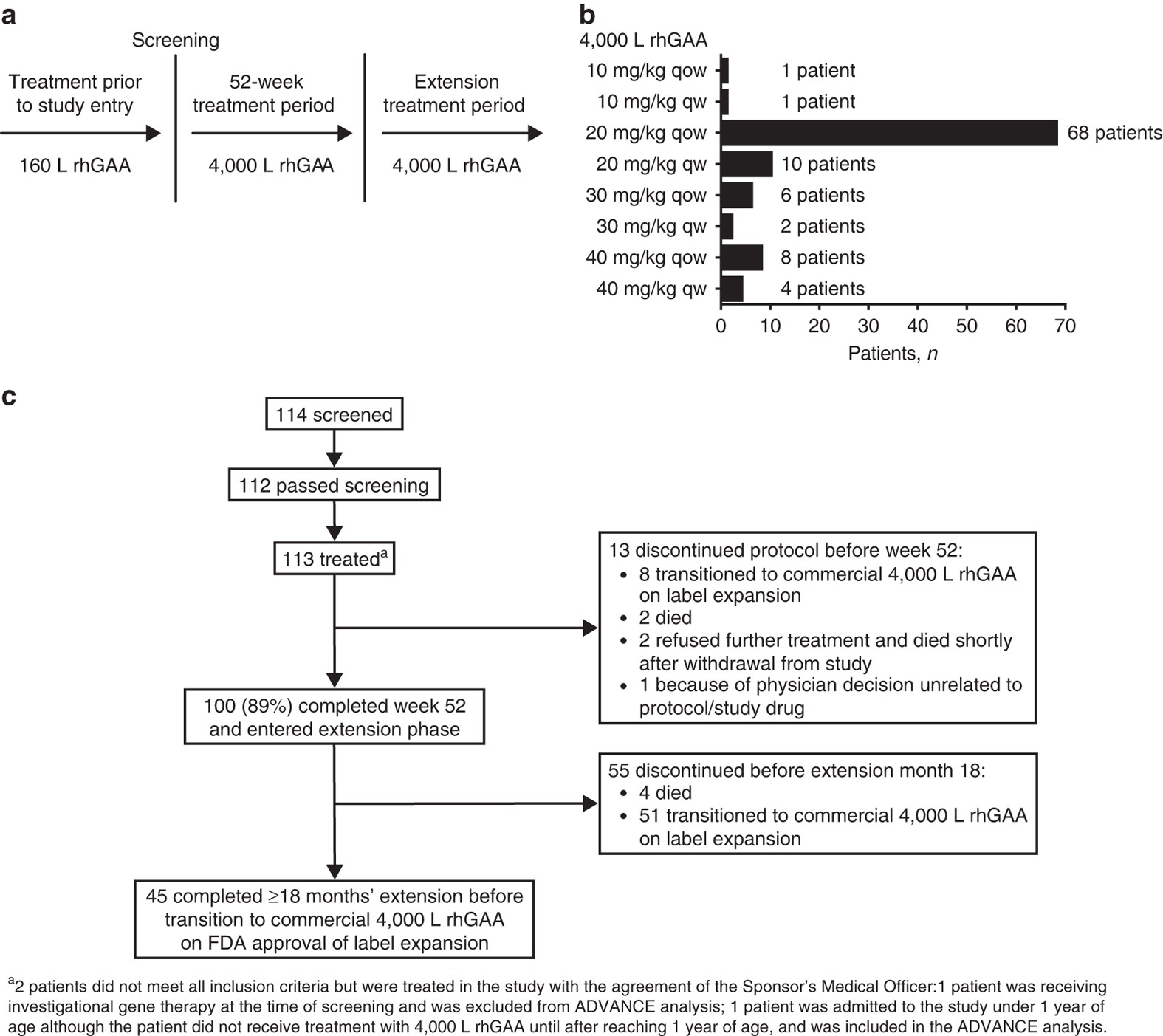 Efficacy Safety Profile And Immunogenicity Of Alglucosidase Alfa
Efficacy Safety Profile And Immunogenicity Of Alglucosidase Alfa
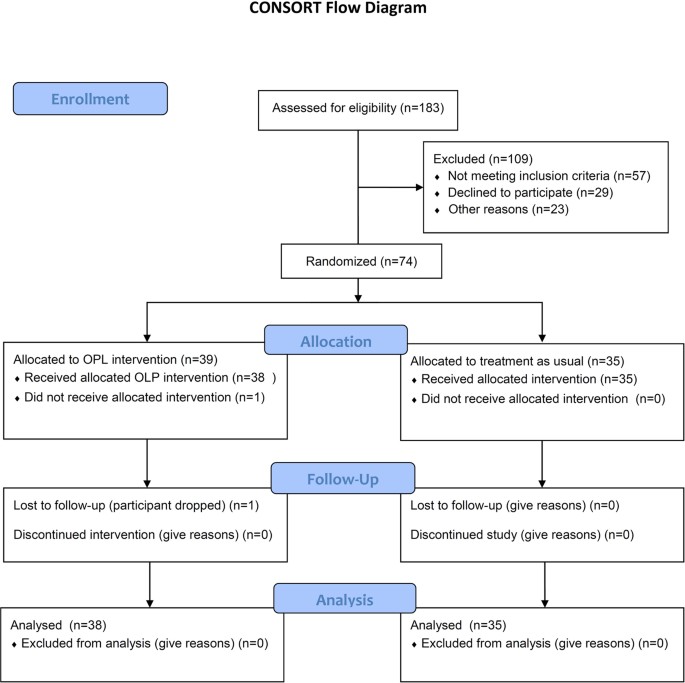 Open Label Placebo Treatment For Cancer Related Fatigue A
Open Label Placebo Treatment For Cancer Related Fatigue A
 Cannabidiol In Patients With Lennox Gastaut Syndrome Interim
Cannabidiol In Patients With Lennox Gastaut Syndrome Interim
 Long Term Efficacy And Safety Of Obeticholic Acid For Patients
Long Term Efficacy And Safety Of Obeticholic Acid For Patients
 Humira Adalimumab Hidradenitis Suppurativa Clinical Data
Humira Adalimumab Hidradenitis Suppurativa Clinical Data
Long Term Efficacy And Safety Of Evolocumab In Patients With
 Psoriatic Arthritis 3 Year Clinical Trial Results Cosentyx
Psoriatic Arthritis 3 Year Clinical Trial Results Cosentyx
 Results From A Long Term Open Label Extension Study Of Adjunctive
Results From A Long Term Open Label Extension Study Of Adjunctive
 Nusinersen In Later Onset Spinal Muscular Atrophy Neurology
Nusinersen In Later Onset Spinal Muscular Atrophy Neurology
 Long Term Safety Of Crisaborole Ointment 2 In Children And Adults
Long Term Safety Of Crisaborole Ointment 2 In Children And Adults
Post a Comment for "31 Open Label Extension Study Definition"