32 Fda Supplement Facts Label Guidelines
These fda food labeling web pages address the labeling requirements for foods under the federal food drug and cosmetic act and its amendments. Ingredient listings should also include the use of spices natural and artificial flavors.
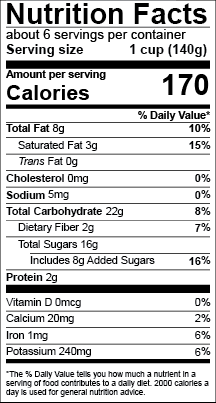 Us Fda Nutrition Facts Labels Food Labeling Software Esha Research
Us Fda Nutrition Facts Labels Food Labeling Software Esha Research
What other information is required.

Fda supplement facts label guidelines. And e on the nutrition and supplement facts. An ingredient statement is needed unless the product ingredients are listed under the supplement facts label. 1 2020 for manufacturers with 10 million or more in annual food sales.
Ingredients are required to be listed in descending order of prominence by weight. Manufacturers with less than 10 million in annual food sales would receive an extra year to complyuntil jan. The dietary supplement health and education act of 1994 the dshea amended the act in part by defining dietary supplements adding specific labeling requirements for dietary supplements and.
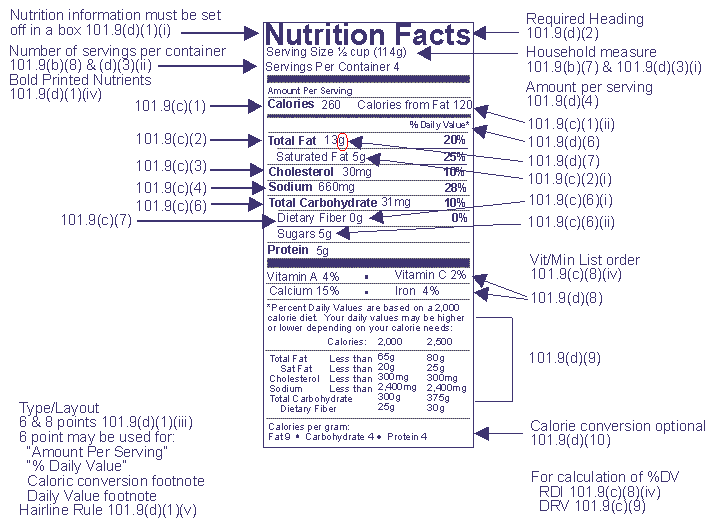
Fda nutrition label font and rule sizes the fdas examples are in helvetica and franklin gothic but any legible font may be used. Eshas genesis rd program uses arial for all formatting. The supplement facts panel on the labels of intermediate sized packages must use type size no smaller than 6 point except that type no smaller than 45 point may be used on packages that have.
You must place the supplement facts panel the ingredient list and the name and place of business of the manufacturer packer or distributor on the information panel if such information does. The fda extended the compliance dates for the nutrition facts and supplement facts label final rule and the serving size final rule from july 26 2018 to january 1 2020 for manufacturers with. All headings and nutrients that are not indented are required to be highlighted in bold or extra bold type.
Nutrition and supplement facts labels questions and answers related to the compliance date added sugars and declaration of quantitative amounts of. 29 fda issued a proposed rule to extend the compliance dates for the updated nutrition facts and supplement facts label final rule and the serving size final rule from july 26 2018 to jan.
Nutrition Facts Panel Amp Label What S New In 2020
 Fda Focuses On Compliance Not Enforcement With Enforcement
Fda Focuses On Compliance Not Enforcement With Enforcement
 New Fda Supplement Facts Box Labeling Changes Thorne S Ahead Of
New Fda Supplement Facts Box Labeling Changes Thorne S Ahead Of
 Keller Heckman Fda Announces Major Revisions To The Nutrition
Keller Heckman Fda Announces Major Revisions To The Nutrition
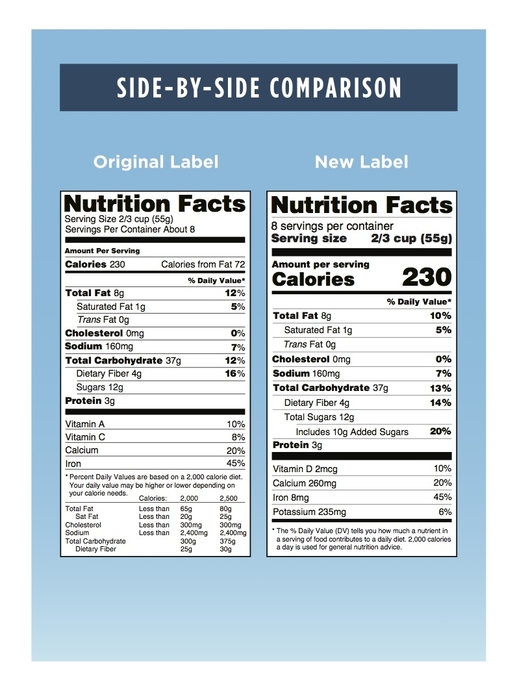 U S Fda Food Beverage And Dietary Supplement Labeling Requirements
U S Fda Food Beverage And Dietary Supplement Labeling Requirements
 How To Comply With Fda Requirements For Dietary Supplement Labeling
How To Comply With Fda Requirements For Dietary Supplement Labeling
 Supplement Facts Labeling Gmp Dietary Label Template Esha Research
Supplement Facts Labeling Gmp Dietary Label Template Esha Research
 The New Fda Nutrition Facts Labels Place More Visual Emphasis On
The New Fda Nutrition Facts Labels Place More Visual Emphasis On
 New Nutrition Facts Label Watson Inc
New Nutrition Facts Label Watson Inc
 How To Comply With Fda Requirements For Dietary Supplement Labeling
How To Comply With Fda Requirements For Dietary Supplement Labeling
 Us Fda Modernizes Nutrition Facts Label For Packaged Foods Sgs
Us Fda Modernizes Nutrition Facts Label For Packaged Foods Sgs
Fda Food And Beverage Labeling Requirements Registrar Corp
 Us Fda Nutrition Facts Labels Food Labeling Software Esha Research
Us Fda Nutrition Facts Labels Food Labeling Software Esha Research
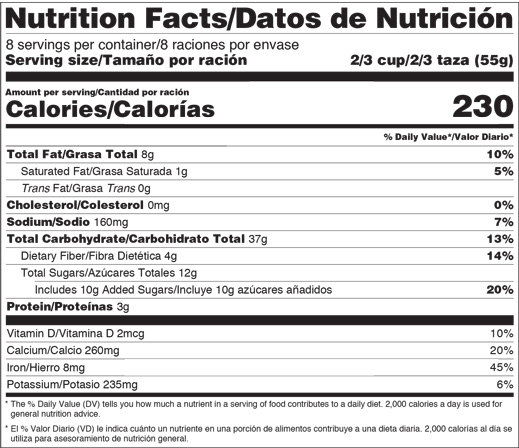 Changes To The Nutrition Facts Label Fda
Changes To The Nutrition Facts Label Fda
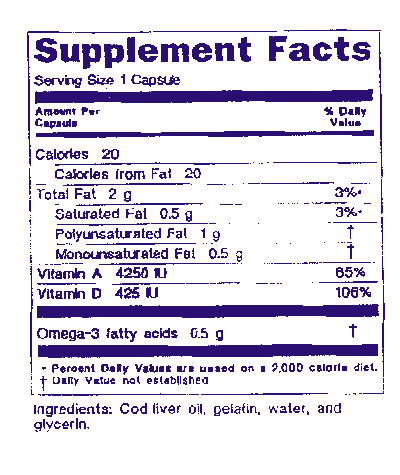 Small Entity Compliance Guide Statement Of Identity Nutrition
Small Entity Compliance Guide Statement Of Identity Nutrition
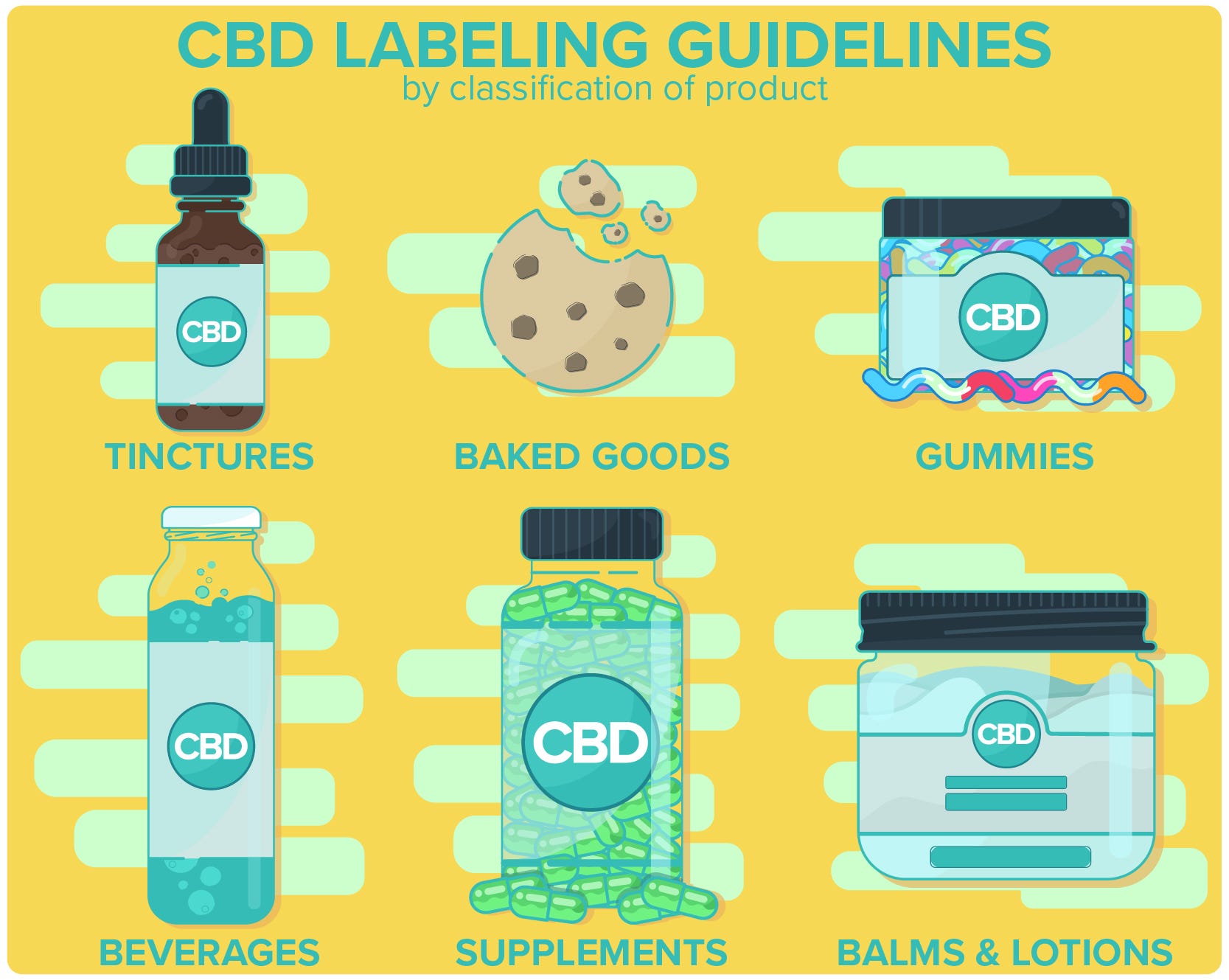 Cbd Labeling Guidelines And Requirements Labelvalue
Cbd Labeling Guidelines And Requirements Labelvalue
 Fda Provides Industry Guidance For Nutrient Conversion Units On
Fda Provides Industry Guidance For Nutrient Conversion Units On
 Navigating The 2019 Fda Label Formatting Rules Labelcalc
Navigating The 2019 Fda Label Formatting Rules Labelcalc
 New Fda Supplement Facts Box Labeling Changes Thorne S Ahead Of
New Fda Supplement Facts Box Labeling Changes Thorne S Ahead Of
 To Prep For Upcoming Supplement Facts Changes Crn Launches Be
To Prep For Upcoming Supplement Facts Changes Crn Launches Be
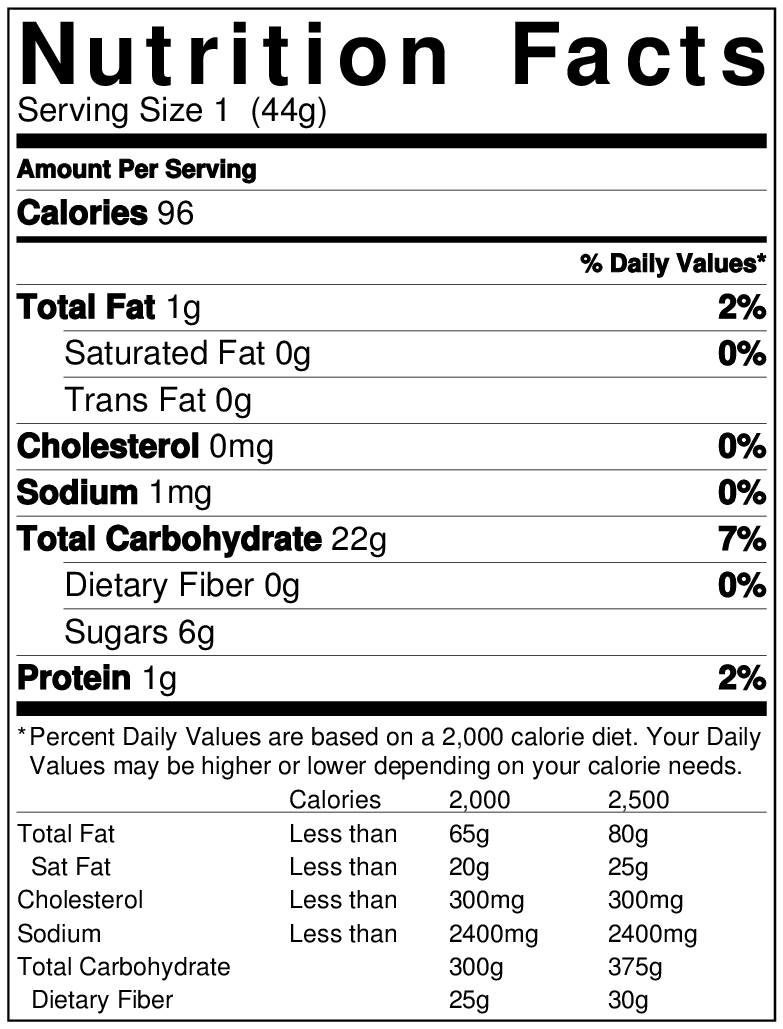 Major Changes Coming To Nutrition Facts And Supplement Facts
Major Changes Coming To Nutrition Facts And Supplement Facts
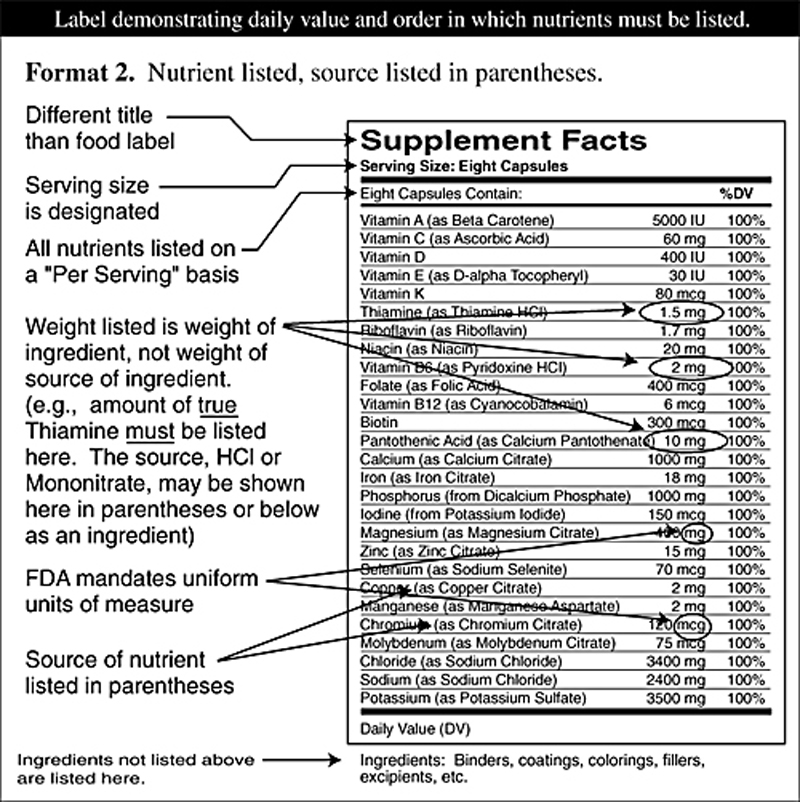 Supplement Facts All The Facts
Supplement Facts All The Facts
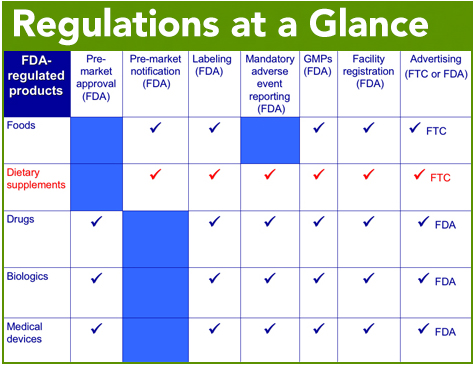 Regulation Council For Responsible Nutrition
Regulation Council For Responsible Nutrition
 Federal Register Food Labeling Revision Of The Nutrition And
Federal Register Food Labeling Revision Of The Nutrition And
 Supplement Facts Background Deception Qualities And Dangers Of
Supplement Facts Background Deception Qualities And Dangers Of
 Fda Grants 6 Month Period Of Enforcement Discretion For Nutrition
Fda Grants 6 Month Period Of Enforcement Discretion For Nutrition
 Federal Register Food Labeling Revision Of The Nutrition And
Federal Register Food Labeling Revision Of The Nutrition And
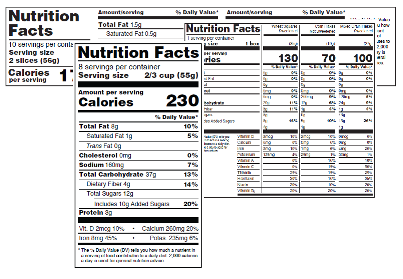 Changes To The Nutrition Facts Label Fda
Changes To The Nutrition Facts Label Fda


Post a Comment for "32 Fda Supplement Facts Label Guidelines"