31 Open Label Study Definition
A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. An open label trial or open trial is a type of clinical trial in which information is not withheld from trial participants.
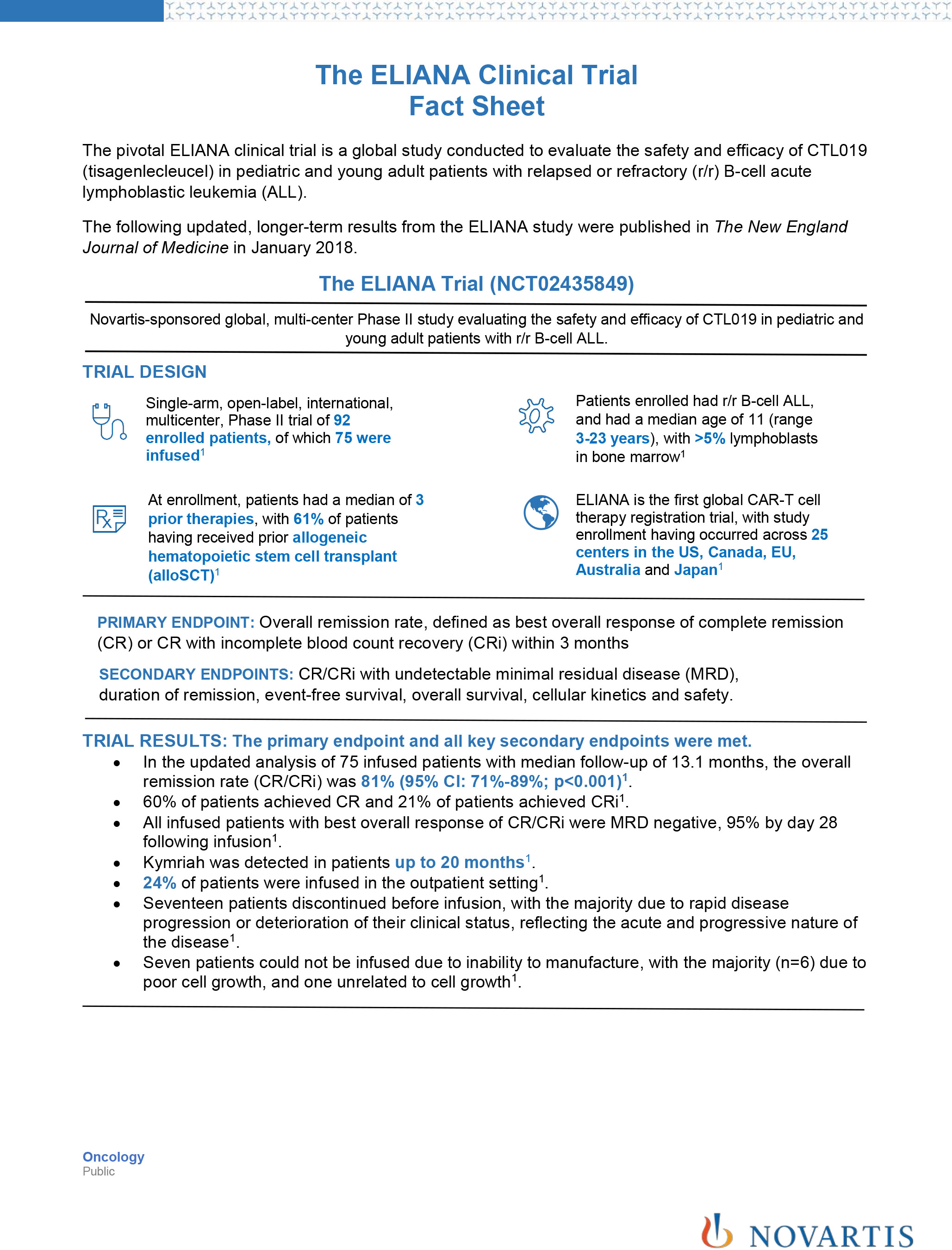 Novartis Announces Nejm Publication Of Updated Analysis From
Novartis Announces Nejm Publication Of Updated Analysis From
The study of language has overturned many misconceptions.

Open label study definition. An open label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. The effort to acquire knowledge as by reading observation or research. Nci dictionary of cancer terms.
In open label trials both the researchers and participants know which drug or other intervention is being given to participants. Open label extension studies allow continued prescribing of unlicensed drugs after a randomised trial but it is unclear whether patients or drug companies are benefiting the most properly designed and conducted open label extension studies can provide rigorous information on long term safety and tolerability of potential new drugs. Open label is the opposite of double blind when neither the researcher nor the participant knows what treatment the participant is receiving.
A type of clinical trial. Open label study listen oh pen lay bel stuh dee a type of study in which both the health providers and the patients are aware of the drug or treatment being given. Medical definition of open label.
In particular both the researchers and participants know which treatment is being administered. A clinical trial without a control group in which both patients and researchers know the identity of the treatment and its dosage. Open label study.
This contrasts with a blinded experiment where information is withheld to reduce bias. Sometimes the circumstances of a trial make it impossible to conceal the identity of the treatment patients are receiving and in other cases researchers may have a specific reason for wanting to use the open label trial format. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects 6 10.
Being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject long term open label studies compare double blind single blind. A clinical study in which the patientssubjects and investigators know which product each patientsubject is receiving which is the opposite of a blinded study. Open label study synonyms open label study pronunciation open label study translation english dictionary definition of open label study.
Many trial designs do not permit blinding and are therefore designed as open label with patients clinicians and other study investigators aware of treatment allocation.
 Denosumab Effective In Refractory Hypercalcemia
Denosumab Effective In Refractory Hypercalcemia
 Pdf Safety And Efficacy Of Olanzapine Monotherapy In Treatment
Pdf Safety And Efficacy Of Olanzapine Monotherapy In Treatment
 Full Text Efficacy Of Long Term Milnacipran Treatment In Patients
Full Text Efficacy Of Long Term Milnacipran Treatment In Patients
 Clinical Trials Information For Ovarian Cancer Treatment
Clinical Trials Information For Ovarian Cancer Treatment
 Phase 1 Open Label Study Of Medi 547 In Patients With Relapsed Or
Phase 1 Open Label Study Of Medi 547 In Patients With Relapsed Or
High Uptake And Reduced Hiv 1 Incidence In An Open Label Trial Of
Conivaptan For The Treatment Of Severe Hyponatremia Open Label
 Placebos Can Work Even When People Know They Re Not Real
Placebos Can Work Even When People Know They Re Not Real
Plos One Impact Of Baseline Bmi On Glycemic Control And Weight
 Primary Care Led Weight Management For Remission Of Type 2
Primary Care Led Weight Management For Remission Of Type 2
Form 8 K X4 Pharmaceuticals Inc For Apr 01
High Uptake And Reduced Hiv 1 Incidence In An Open Label Trial Of
The Crystal Study A 24 Month Phase Iiib Open Label Single Arm
 Pdf Open Label Study Of Long Term Administration Of Dotinurad In
Pdf Open Label Study Of Long Term Administration Of Dotinurad In
 Placebo Controlled Trials For Mesothelioma
Placebo Controlled Trials For Mesothelioma
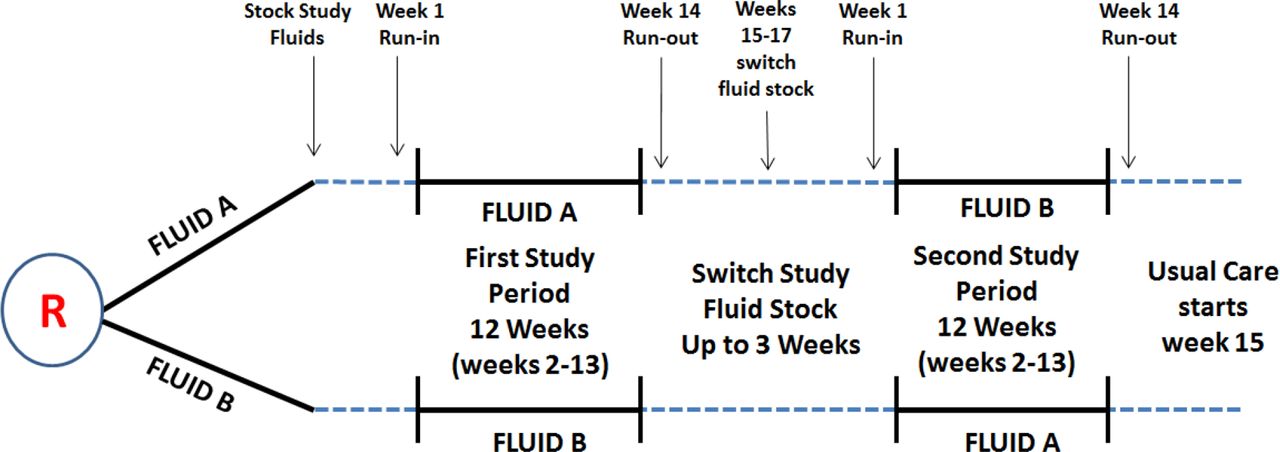 Fluid Trial A Protocol For A Hospital Wide Open Label Cluster
Fluid Trial A Protocol For A Hospital Wide Open Label Cluster

 How To Process Data From Clinical Trials And Their Open Label
How To Process Data From Clinical Trials And Their Open Label
 Solved A 10 Points In The Abstract For This Stud
Solved A 10 Points In The Abstract For This Stud
 Cctg 595 A Multicenter Randomized Study Of Text Messaging To Improv
Cctg 595 A Multicenter Randomized Study Of Text Messaging To Improv
 Clovis Oncology Inc Is Discontinuing Its Sponsored Phase 2 Open
Clovis Oncology Inc Is Discontinuing Its Sponsored Phase 2 Open
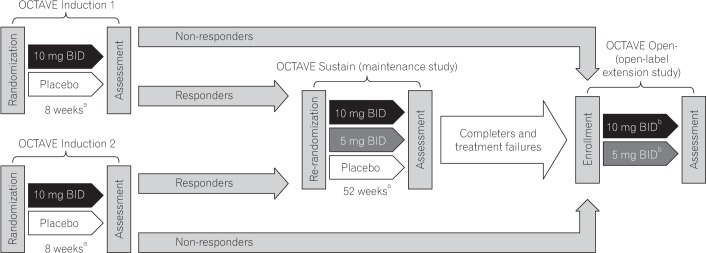 Tofacitinib Induction And Maintenance Therapy In East Asian
Tofacitinib Induction And Maintenance Therapy In East Asian
Form 8 K Ovid Therapeutics Inc For Mar 11
 Randomized Controlled Trial Wikipedia
Randomized Controlled Trial Wikipedia
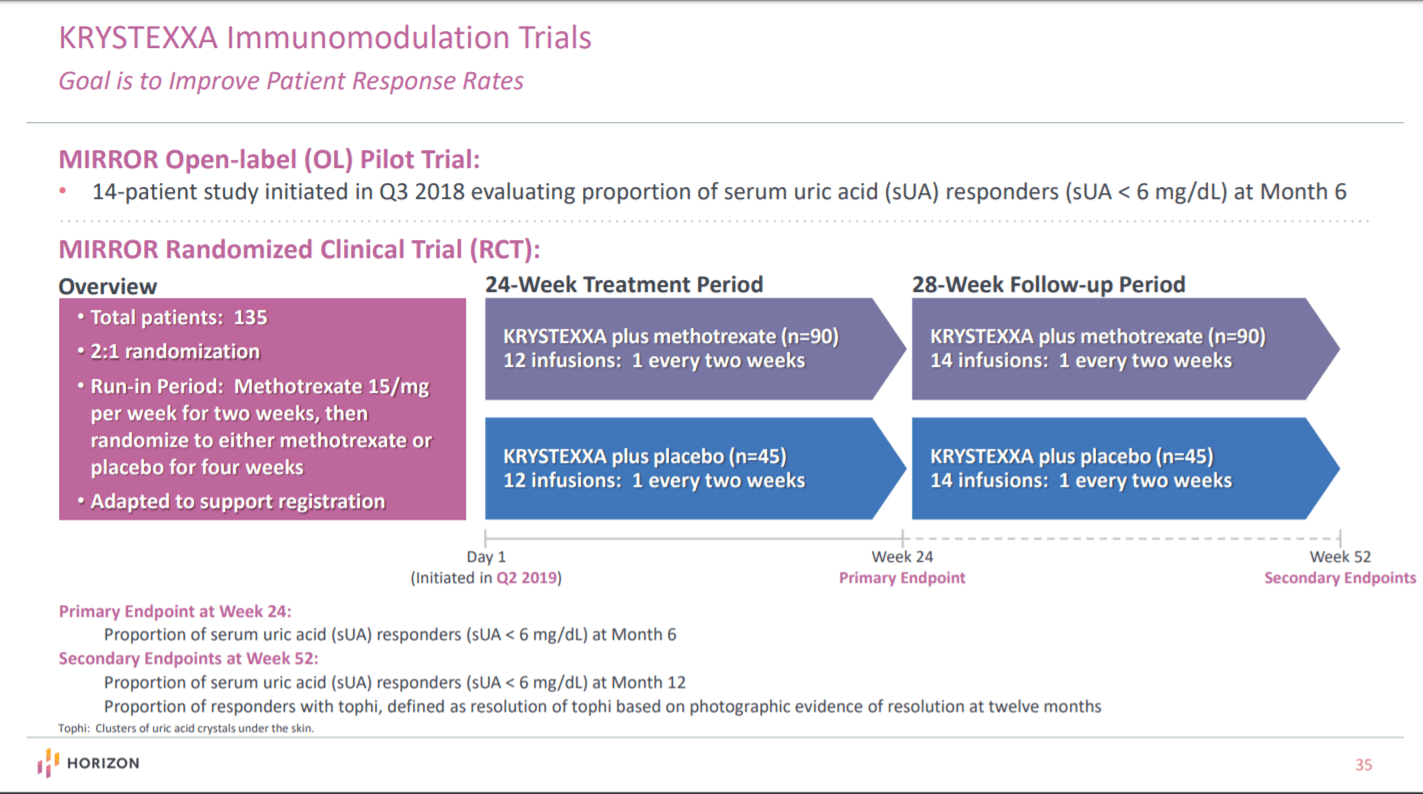 Horizon Therapeutics Looking Solid In 2020 Horizon Therapeutics
Horizon Therapeutics Looking Solid In 2020 Horizon Therapeutics
An Open Label Pilot Study To Assess The Efficacy And Safety Of
 Real World Data And Evidence Revolutionize Clinical Research Sprim
Real World Data And Evidence Revolutionize Clinical Research Sprim
 Tom Walker On Twitter Pretty Much Ticks The Consort Guidelines
Tom Walker On Twitter Pretty Much Ticks The Consort Guidelines
 A Phase 3 Trial Of Sebelipase Alfa In Lysosomal Acid Lipase
A Phase 3 Trial Of Sebelipase Alfa In Lysosomal Acid Lipase
Post a Comment for "31 Open Label Study Definition"