34 Extra Label Drug Use Definition
Extra label drug use revisited. A resource for veterinarians.
 Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
Similarly fda is proposing to ban the extra label use of cephalosporins as well adding this class of drugs to the prohibited list.
Extra label drug use definition. Off label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group dosage or route of administration. Using the drug according to the empirical method d. Extra label drug use whether actual or intended occurs when the drug is used in a manner not in accordance with approved label directions.
Deciding how long the drug should be given. What you need to know. Otherwise the drug is being used in an extra label manner.
Food and drug administration fda recently approved baytril for use in swine but prohibited any extra label use. Prior to the passage. July 9 2008 the us.
Off label use is generally legal unless it violates ethical guidelines or safety regulations. Reference is also often made to off label use where an unapproved drug is used in a manner. Extra label use means.
Extra label use elu is defined as the use of a drug product in a manner that is not consistent with what is indicated on the label package insert or product monograph of any drug product approved by health canada. The ins and outs of extra label drug use in animals. The animal medicinal drug use clarification act of 1994 amduca made eldu an fda regulated veterinary medical activity allowing veterinarians to prescribe extra label uses of approved animal and human drugs when the health of an animal is threatened or when suffering or death may result from failure to treat animals.
Using a drug in a way not specified by the label c. As a practicing veterinarian youve likely prescribed a drug for an extra label use. Both prescription drugs and over the counter drugs otcs can be used in off label ways although most studies of off label use focus on prescription drugs.
Prescription drugs are often prescribed for uses other than what the fda has approved. To use an nad in a legal manner veterinarians must adhere to the specifications noted on the label.
 What Is Capital Definition Types And Structure Thestreet
What Is Capital Definition Types And Structure Thestreet
 5 Pack Identify Diagnostics 10 Panel Drug Test Cup Testing Instantly For 10 Different Drugs Thc Coc Oxy Mop Amp Bar Bzo Met Mtd Pcp Id Cp10
5 Pack Identify Diagnostics 10 Panel Drug Test Cup Testing Instantly For 10 Different Drugs Thc Coc Oxy Mop Amp Bar Bzo Met Mtd Pcp Id Cp10
 2018 National Survey On Drug Use And Health Methodological
2018 National Survey On Drug Use And Health Methodological
 Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
 Veterinary Antidotes And Availability An Update Pdf Free Download
Veterinary Antidotes And Availability An Update Pdf Free Download
 Off Label Drug Use Why Do Doctors Prescribe Them
Off Label Drug Use Why Do Doctors Prescribe Them
 Silver Sulfadiazine Equimed Horse Health Matters
Silver Sulfadiazine Equimed Horse Health Matters
 Proper Usage Of Drugs Chemicals And Feed Additives In Market Show
Proper Usage Of Drugs Chemicals And Feed Additives In Market Show

 Pdf Commonly Used Drugs Uses Side Effects Bioavailability
Pdf Commonly Used Drugs Uses Side Effects Bioavailability

Inappropriate Usage Of Selected Antimicrobials Comparative
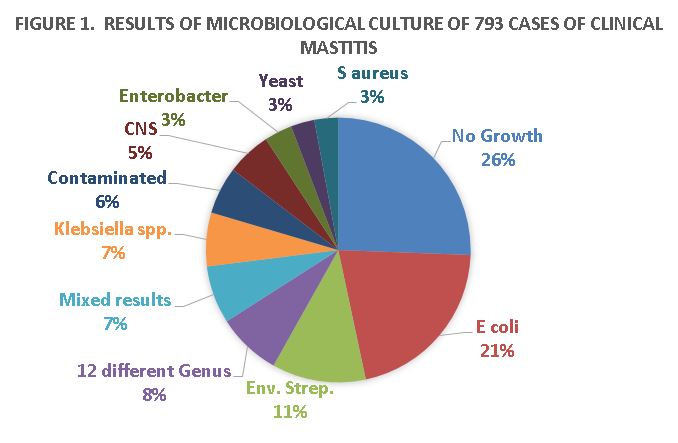 Responsible Use Of Antibiotics For Treatment Of Clinical Mastitis
Responsible Use Of Antibiotics For Treatment Of Clinical Mastitis
 Fact Check Does Zantac Cause False Positives For Methamphetamines
Fact Check Does Zantac Cause False Positives For Methamphetamines
 Dr Christine Hoang Legislative Regulatory Update Congress Activit
Dr Christine Hoang Legislative Regulatory Update Congress Activit
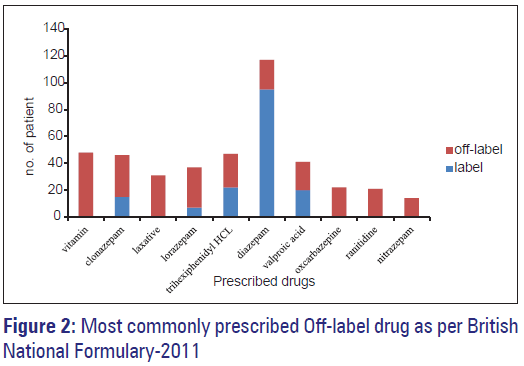 Off Label Drug Use In Psychiatry Outpatient Department A
Off Label Drug Use In Psychiatry Outpatient Department A
 Safety Labels Vs Signs What S The Difference Ehs Today
Safety Labels Vs Signs What S The Difference Ehs Today
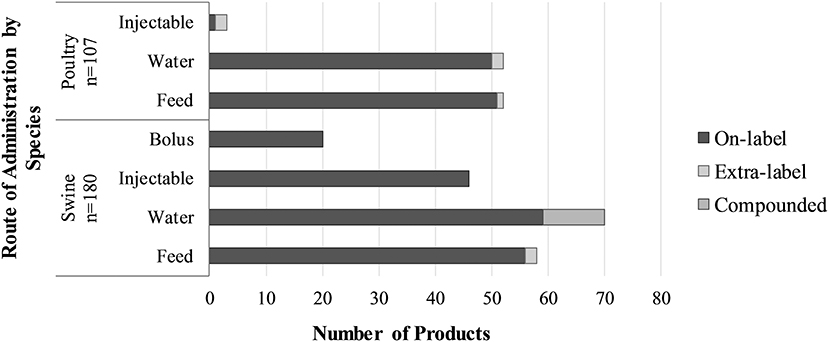 Frontiers Developing Canadian Defined Daily Doses For Animals A
Frontiers Developing Canadian Defined Daily Doses For Animals A
 America S War On Pain Pills Is Killing Addicts And Leaving
America S War On Pain Pills Is Killing Addicts And Leaving
Executive Summary The Use Of Drugs In Food Animals Benefits And
 Off Label Drug Use Why Do Doctors Prescribe Them
Off Label Drug Use Why Do Doctors Prescribe Them
Qualified Individual And Licensed Retailer Resources Farad S
Special Topics Farad S Species Pages
 Students Used To Take Drugs To Get High Now They Take Them To Get
Students Used To Take Drugs To Get High Now They Take Them To Get
 Going Off Label Using Type 2 Diabetes Drugs For T1d
Going Off Label Using Type 2 Diabetes Drugs For T1d
 Do I Need A Vet For My Bees Bee Culture
Do I Need A Vet For My Bees Bee Culture
 What Do You Think Quality Assurance Means Quality Assurance Image
What Do You Think Quality Assurance Means Quality Assurance Image
 Improper Drug Dosage Wrong Medication Interactions With Other
Improper Drug Dosage Wrong Medication Interactions With Other
 Drug Action And Pharmacodynamics Pharmacology Merck Veterinary
Drug Action And Pharmacodynamics Pharmacology Merck Veterinary
 Levorphanol Tartrate Tablets Usp Cii
Levorphanol Tartrate Tablets Usp Cii



Post a Comment for "34 Extra Label Drug Use Definition"