32 Fda Drug Label Requirements
An example of fda approved labeling is the professional package insert ppi. Registrar corp can review your drugs labeling for compliance with us.
 Toxtutor Regulation Of Consumer Products And Drug Safety
Toxtutor Regulation Of Consumer Products And Drug Safety
Fdas guidance for industry entitled help seeking and other disease awareness communications by or on behalf of drug and device firms january 2004 describes two types of drug labeling.

Fda drug label requirements. 20125 bar code label requirements. These fda food labeling web pages address the labeling requirements for foods under the federal food drug and cosmetic act and its amendments. 20124 labeling for systemic antibacterial drug products.
Among other aspects fda requires otc drug labels to list active ingredients drug uses specific warnings and dosage instructions. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription drug and biological products under 21 cfr 20156d and 20157. 20126 exceptions or alternatives to labeling requirements for human drug products held by the strategic national stockpile.
All drugs distributed in the united states must comply with the federal food drug and cosmetic act fdca regardless of where they are made. Subpart b labeling requirements for prescription drugs andor insulin 20150 statement of identity. The goal of the physician labeling rule content and format requirements as described at 21 cfr 20156 and 20157 is to enhance the safe and effective use of prescription drug products by providing.
Fda approved labeling and promotional labeling. Drug labeling and ingredient reviews. The fdca and fda regulations require that drugs meet manufacturing standards to assure quality and compliance with drug label requirements.
 Peter Grossi Fda 39 S Plan To Require Generic Companies To Modify Drug
Peter Grossi Fda 39 S Plan To Require Generic Companies To Modify Drug
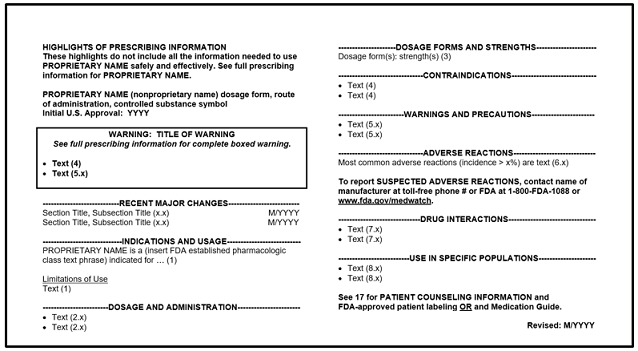 Prescription Drug Labeling Resources Fda
Prescription Drug Labeling Resources Fda

 2 Labeling For Human Prescription Drug And Biological Products
2 Labeling For Human Prescription Drug And Biological Products
 A Comparison Of Fda And Ema Drug Approval Implications For Drug
A Comparison Of Fda And Ema Drug Approval Implications For Drug
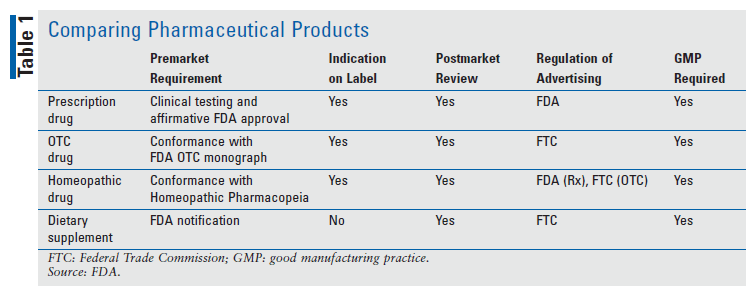 Lesson Reviewing The Regulatory Landscape
Lesson Reviewing The Regulatory Landscape
 Standardization Of Labeling Requirements Board Of Pharmacy
Standardization Of Labeling Requirements Board Of Pharmacy
 Fda Draft Guidance On Responding To Unsolicited Requests For Off Labe
Fda Draft Guidance On Responding To Unsolicited Requests For Off Labe
![]() Regulatory Affairs Archives Page 4 Of 5 Pdg
Regulatory Affairs Archives Page 4 Of 5 Pdg
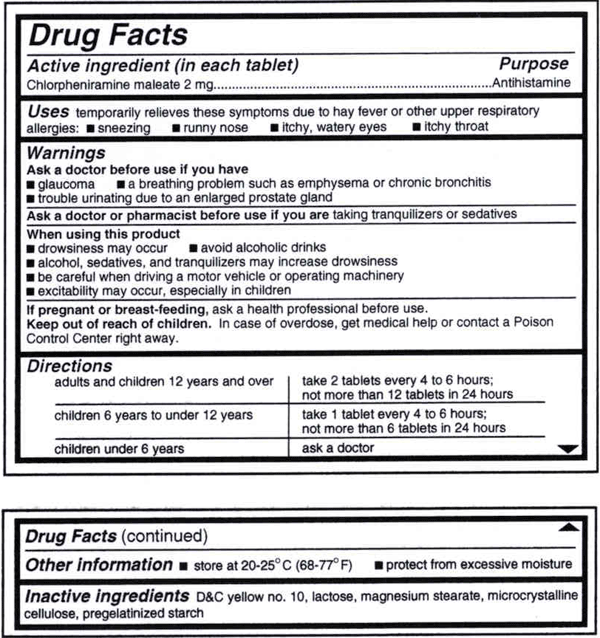
 The Food And Drug Administration Fda Approved Drug Label For
The Food And Drug Administration Fda Approved Drug Label For
Cfr Code Of Federal Regulations Title 21
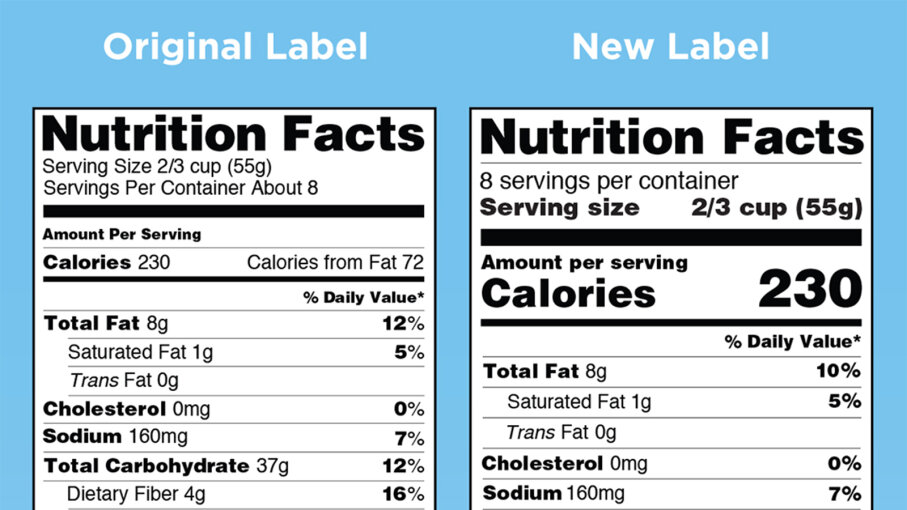 Fda Unveils New Food Nutrition Labels Howstuffworks
Fda Unveils New Food Nutrition Labels Howstuffworks
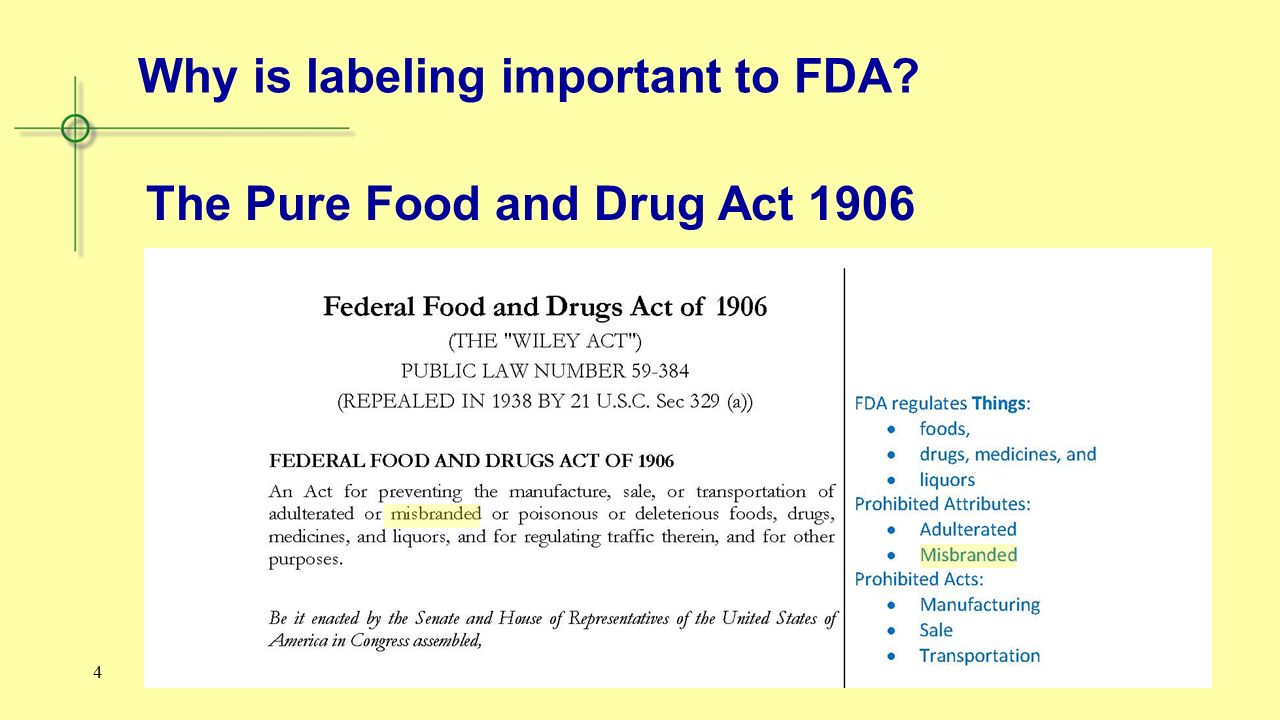 Fda Labeling And Marketing Ppt Video Online Download
Fda Labeling And Marketing Ppt Video Online Download
 Book 7 2019 Selected Laws Regulations Guidance On Drug Marketing Advertising And Labeling
Book 7 2019 Selected Laws Regulations Guidance On Drug Marketing Advertising And Labeling
Menu Calorie Labeling Requirements In Supermarkets Iddba
Changes Being Effected To Drug Labeling Regime Fda Releases
 Beyond The Fda Pro Guidance Steps Toward Integrating Meaningful
Beyond The Fda Pro Guidance Steps Toward Integrating Meaningful
 Webinar Fda Drug Listing Requirements For Manufacturers
Webinar Fda Drug Listing Requirements For Manufacturers
 Food And Drug Administration Fda
Food And Drug Administration Fda
 The Fda Bows To Industry Pressure On Added Sugar Labeling Fooducate
The Fda Bows To Industry Pressure On Added Sugar Labeling Fooducate
.jpg) Fda Drug Labeling And Ingredient Requirement Fda Registration
Fda Drug Labeling And Ingredient Requirement Fda Registration
 Fda 2013 Clinical Investigator Training Course Cmc And Investigator
Fda 2013 Clinical Investigator Training Course Cmc And Investigator
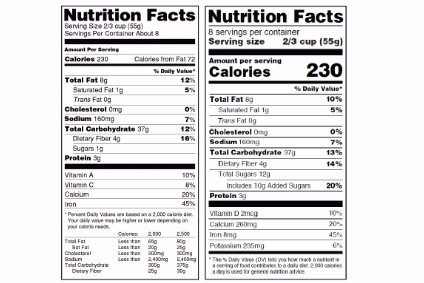 Us Government Provides Further Guidance On Nutrition Facts Label
Us Government Provides Further Guidance On Nutrition Facts Label
 Us Fda Modernizes Nutrition Facts Label For Packaged Foods Sgs
Us Fda Modernizes Nutrition Facts Label For Packaged Foods Sgs
Investigational Drugs Strategies For Sponsors Fda And Clinical
 Peter Grossi Fda 39 S Plan To Require Generic Companies To Modify Drug
Peter Grossi Fda 39 S Plan To Require Generic Companies To Modify Drug
 Statistics For Clinical Trial Announcements Cta And Fda Drug
Statistics For Clinical Trial Announcements Cta And Fda Drug
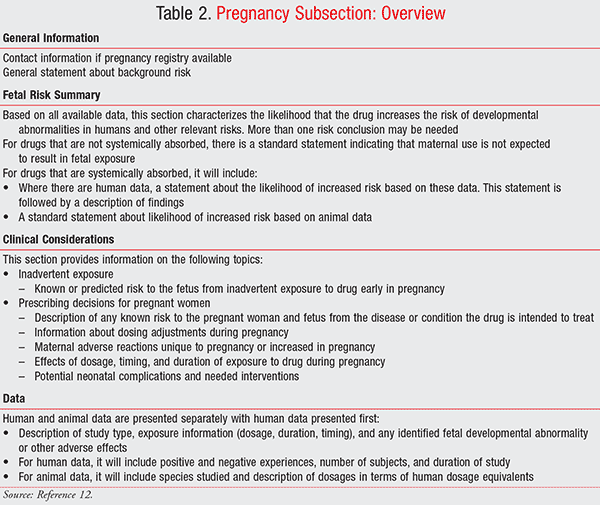 New Changes In Pregnancy And Lactation Labeling
New Changes In Pregnancy And Lactation Labeling


Post a Comment for "32 Fda Drug Label Requirements"