30 Which Of The Following Is Not A Requirement For A Container Label_
Which of the following medication does not require that a patient package insert be given to the patient. What type of container is impervious to air under normal handling shipment storage and distribution.
 Exemption From Nutrition Labeling Requirements Fda Reader
Exemption From Nutrition Labeling Requirements Fda Reader
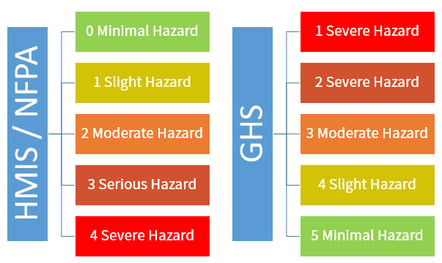
When two or more different primary and subsidiary hazard labels are required they must be displayed.
Which of the following is not a requirement for a container label_. See example 2 labels must be legible in english and prominently displayed. The 6 main elements of a ghs label ghs compliant labels contain six main elements. The rest of the necessary information can be determined by the employer.
If in doubt label it. All of the above require a marine pollutant marking. The outer container does not require a whmis label if the label on the inner container is visible and legible through the outer container under normal conditions of storage and handling or the outer container has a label that meets the requirements set out in the transportation of dangerous goods regulations.
While the dot diamond label is required for all hazardous chemicals on the outside shipping containers chemicals in smaller containers inside the larger shipped container do not require the dot diamond but do require the osha pictograms. One common case where you do not have to label a secondary container is if the container is portable and will be used immediately by the person who transferred the chemical into that container. Like the dusty binder on the top shelf a container with a piece of curled masking tape that has something written in smudged ballpoint pen probably is not going to satisfy a compliance officer.
The old primary container label requirements. The employer is not required to label portable containers. As you can see from the regulation the only specific requirement is that that label include the product identifier.
Except for a few cases secondary containers must be labeled. Note that these requirements apply to primary containers which includes the containers received from the manufacturer but not specifically to secondary containers such as smaller jars or spray bottles that hold chemicals transferred from the primary container. Portable containers are used to transfer hazardous chemicals from labeled containers and are intended only for the immediate use by the employee who performs the transfer.
When is the marine pollutant marking required on a container. One of the facets of a complete hazard communication program is establishing a process for proper container labeling. The rest of the necessary information can be determined by the employer.
Which of the following is not required on a medication order label. Most containers shipped directly from the manufacturer or purchased from a distributor are called shipped or primary containerslabeling information on these containers is usually adequate in communicating the hazards of the chemical. A hazard label is not required for which of these types of packages.
 Fda Grants 6 Month Period Of Enforcement Discretion For Nutrition
Fda Grants 6 Month Period Of Enforcement Discretion For Nutrition
 Recreational Marijuana Packaging And Labeling Pre Approval
Recreational Marijuana Packaging And Labeling Pre Approval
 Ghs Compliant Labels What Are The Essential Components
Ghs Compliant Labels What Are The Essential Components
Container Labels Chemical Hazards Ehs Uw Superior
 Regulatory And Public Influences Ba 850 Sustainability Driven
Regulatory And Public Influences Ba 850 Sustainability Driven
What Required Information Must Ghs Labels Include Mpc
 Secondary Container Labels 101 Hazcom And Whmis
Secondary Container Labels 101 Hazcom And Whmis
 Amazon Com Globally Harmonized System Ghs Secondary Container
Amazon Com Globally Harmonized System Ghs Secondary Container
 Empty Research Container Management
Empty Research Container Management
Global Harmonized System A Close Look Grainger Industrial Supply

Ghs Container Labels Review What Manufacturers Importers And
 Requirements For Shipped Container And Workplace Labels Grainger
Requirements For Shipped Container And Workplace Labels Grainger
 Chemical Labeling Chemicals Management Guide Amp Training For
Chemical Labeling Chemicals Management Guide Amp Training For
 Amazon Com Medvalue Ghs Container Label 3 X 1 1 2 Health
Amazon Com Medvalue Ghs Container Label 3 X 1 1 2 Health
 What Are The 6 Elements Of A Ghs Label
What Are The 6 Elements Of A Ghs Label
 Safety Tip Of The Week Laboratory Safety Labeling And Transfer
Safety Tip Of The Week Laboratory Safety Labeling And Transfer
 Dangerous Goods 5 Tipps For Shipping Hazardous Goods
Dangerous Goods 5 Tipps For Shipping Hazardous Goods
 Federal Hazardous Substances Act Fhsa Requirements Cpsc Gov
Federal Hazardous Substances Act Fhsa Requirements Cpsc Gov
 How To Label Your Products For Amazon Fba Onlinelabels Com
How To Label Your Products For Amazon Fba Onlinelabels Com
 Rules For Proper Secondary Container Labeling Vivid Learning Systems
Rules For Proper Secondary Container Labeling Vivid Learning Systems

 Sign Marking Requirements Grainger Industrial Supply
Sign Marking Requirements Grainger Industrial Supply
 Suffocation Warnings Packaging Requirements Legal Guidelines To
Suffocation Warnings Packaging Requirements Legal Guidelines To
Xtendimax Herbicide With Vaporgrip Technology
 Final Gmo Labeling Rule Does Not Require Labeling Of Highly
Final Gmo Labeling Rule Does Not Require Labeling Of Highly
Cfr Code Of Federal Regulations Title 21
Container Labels Secondary Container Labeling Osha Requirements
 Nutrition Labels 101 What S Required What S Optional
Nutrition Labels 101 What S Required What S Optional
Post a Comment for "30 Which Of The Following Is Not A Requirement For A Container Label_"