34 Open Label Study Advantages And Disadvantages
This contrasts with a blinded experiment where information is withheld to reduce bias. Nci dictionary of cancer terms.
 The Application Of Car T Cell Therapy In Hematological
The Application Of Car T Cell Therapy In Hematological
Sometimes the circumstances of a trial make it impossible to conceal the identity of the treatment patients are receiving and in other cases researchers may have a specific reason for wanting to use the open label trial format.

Open label study advantages and disadvantages. An open label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Better worse or just different.
Open label is the opposite of double blind when neither the researcher nor the participant knows what treatment the participant is receiving. Escitalopram in clinical practice. Open label extension studies do have a legitimate but limited place in the clinical development of new medicines.
Results of an open label trial in outpatients with depression in a naturalistic setting in germany. Consumers institutions where these studies are conducted and research ethics committees need to be convinced of the motives as well as the quality of the open label extension study and its execution before supporting such studies. Open label trials are sometimes referred to as non masked or unblinded if the trial is a non pharmacological study such as a trial of devices or psychological and physical treatments it may be referred to simply as open after recruitment to the trial the participants were allocated to treatment using block randomisation.
Möller hj langer s schmauss m. Advantages and disadvantages of using an active control or placebo in clinical studies. Open label study listen oh pen lay bel stuh dee a type of study in which both the health providers and the patients are aware of the drug or treatment being given.
An open label trial or open trial is a type of clinical trial in which information is not withheld from trial participants. Consequence an intense discussion of the advantages and disadvantages of open label or double blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In particular both the researchers and participants know which treatment is being administered.
An intense discussion of the advantages and disadvantages of both designs is currently under way and. In general a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the. External and internal validity of open label or doubleblind trials in oral anticoagulation.
While dabigatran was tested in af using an openlabel study and in acute deep vein. Advantages and disadvantages.
 Assessment Of Clinical Response Devita Hellman And Rosenberg S
Assessment Of Clinical Response Devita Hellman And Rosenberg S
 Randomized Controlled Trial Wikipedia
Randomized Controlled Trial Wikipedia
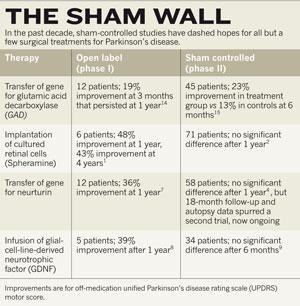 Experimental Therapies For Parkinson S Disease Why Fake It Nature
Experimental Therapies For Parkinson S Disease Why Fake It Nature
 Observation Research Sage Research Methods
Observation Research Sage Research Methods
 10 Advantages And Disadvantages Of Questionnaires Survey Anyplace
10 Advantages And Disadvantages Of Questionnaires Survey Anyplace
The Efficacy Of Dihydroartemisinin Piperaquine And Artemether
 Advantages And Disadvantages Of Available Monitoring Technologies
Advantages And Disadvantages Of Available Monitoring Technologies
 Drop Down Usability When You Should And Shouldn T Use Them
Drop Down Usability When You Should And Shouldn T Use Them
 Questionnaire Design Sage Research Methods
Questionnaire Design Sage Research Methods
 10 Advantages And Disadvantages Of Questionnaires Survey Anyplace
10 Advantages And Disadvantages Of Questionnaires Survey Anyplace
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
 Management Of Type 2 Diabetes Selecting Amongst Available
Management Of Type 2 Diabetes Selecting Amongst Available
 One Way Communication Definition Advantages Amp Examples Video
One Way Communication Definition Advantages Amp Examples Video
 Advantages And Disadvantages Of Mesenchymal Stem Cells Mscs From
Advantages And Disadvantages Of Mesenchymal Stem Cells Mscs From
 Design Characteristics Risk Of Bias And Reporting Of Randomised
Design Characteristics Risk Of Bias And Reporting Of Randomised
 7 Intriguing Native Advertising Advantages And Disadvantages
7 Intriguing Native Advertising Advantages And Disadvantages
 Design Characteristics Risk Of Bias And Reporting Of Randomised
Design Characteristics Risk Of Bias And Reporting Of Randomised
 Advantages And Disadvantages To A Double Major In College Career
Advantages And Disadvantages To A Double Major In College Career
 7 Intriguing Native Advertising Advantages And Disadvantages
7 Intriguing Native Advertising Advantages And Disadvantages
 Summary Of Advantages And Disadvantages Between Microarray And Rna
Summary Of Advantages And Disadvantages Between Microarray And Rna
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
Updates In The Systemic Treatment Of Hepatocellular Carcinoma
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
 20 Advantages And Disadvantages Of Outsourcing From Your Small
20 Advantages And Disadvantages Of Outsourcing From Your Small
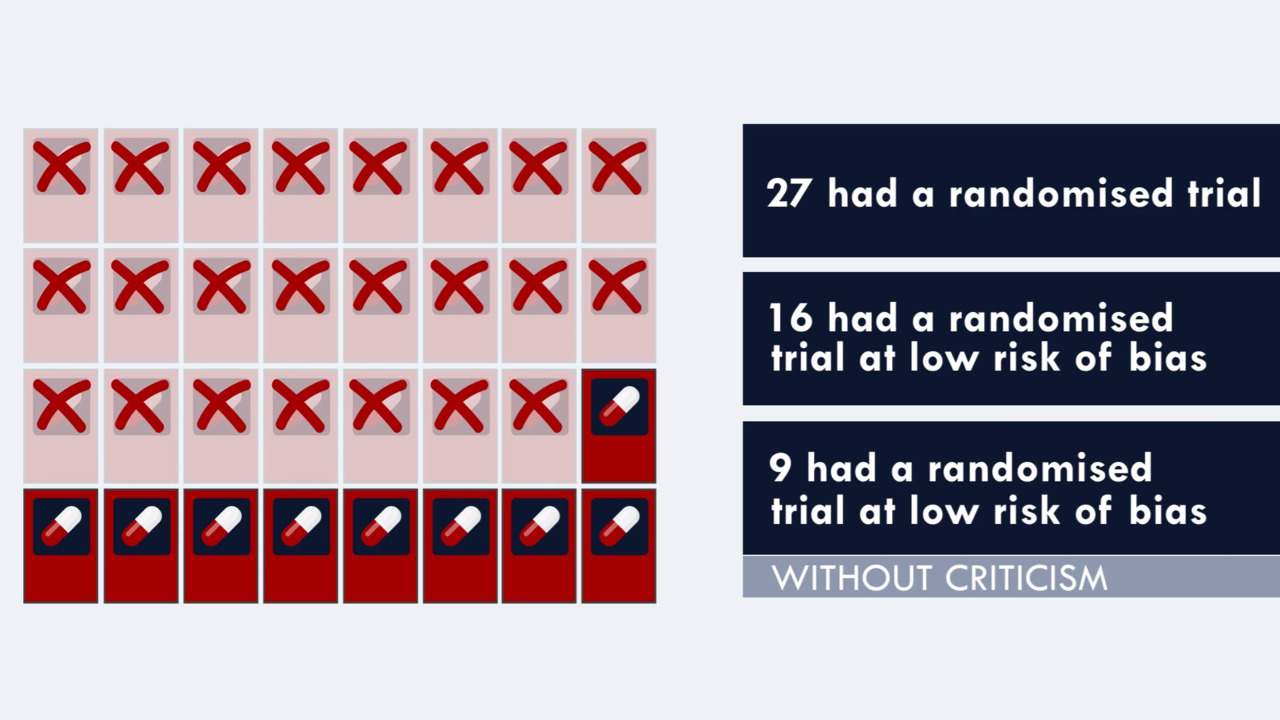 Design Characteristics Risk Of Bias And Reporting Of Randomised
Design Characteristics Risk Of Bias And Reporting Of Randomised
 Targeting Tyrosine Kinase Receptors Pitfalls And Benefits
Targeting Tyrosine Kinase Receptors Pitfalls And Benefits
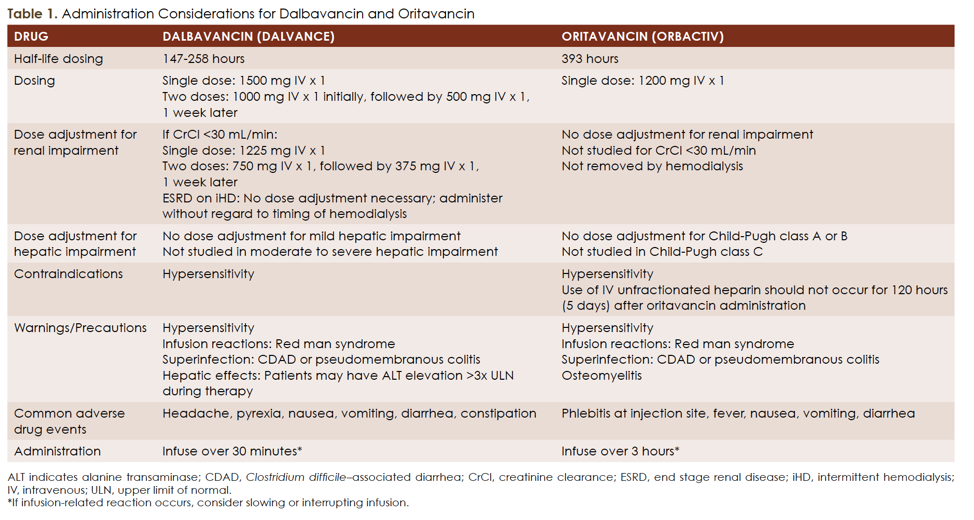 Long Acting Anti Mrsa Agents One Dose To Cure
Long Acting Anti Mrsa Agents One Dose To Cure
 Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
Consort Flowchart For A Single Arm Open Label Phase 2 Clinical
 Everything You Need To Know About Single Blind Peer Review
Everything You Need To Know About Single Blind Peer Review



Post a Comment for "34 Open Label Study Advantages And Disadvantages"