34 Extra Label Drug Use
It also includes the use of all unapproved drugs including unapproved bulk active pharmaceutical ingredients apis. Extra label use elu is defined as the use of a drug product in a manner that is not consistent with what is indicated on the label package insert or product monograph of any drug product approved by health canada.
 Estimating Provisional Acceptable Residues For Extralabel Drug Use
Estimating Provisional Acceptable Residues For Extralabel Drug Use
Food and drug administration fda regulations.

Extra label drug use. A resource for veterinarians. Elu of new animal drugs was considered illegal and permitted. Identity of treated animal is carefully maintained 8.
Eldu describes the use of an approved drug in a manner that is not in accordance with the approved labeling yet meets the conditions set forth by the animal medicinal drug use clarification act of 1994 amduca and us. Extra label drug use refers to the use of an approved drug in a manner that is not in accordance with the approved label directions. Fda may prohibit the extra label use of an animal or human drug in nonfood producing animals if fda determines that such extra label use presents a risk to the public health.
There are few drugs for use in goats that have food and drug administration fda approval. Restrictions regarding extra label drug use of nonfood animal and human drugs are progressively restrictive. Start studying extra label drug use and compounding.
Extra label drug use eldu in animals. Lengthened time period for drug withdraw 7. Drug properly labeled for instructed use by vet 9.
Learn vocabulary terms and more with flashcards games and other study tools. Eldu also referred to as off label use refers to the use or intended use of a drug approved by health canada in an animal in a manner not in accordance with the label or package insert. Administering any drug not specifically labeled for use in goats or any product either prescription or over the counter that is not used as directed on the label is considered extra label or off label drug use.
Extra label drug use in food animals is permitted only by or under the supervision of a veterinarian is allowed only for therapeutic purposes ie. Reference is also often made to off label use where an unapproved drug is used in a manner. What is extra label drug use eldu.
As a practicing veterinarian youve likely prescribed a drug for an extra label use. Extra label drug use of feed additives is not permitted under any circumstances. The ins and outs of extra label drug use in animals.
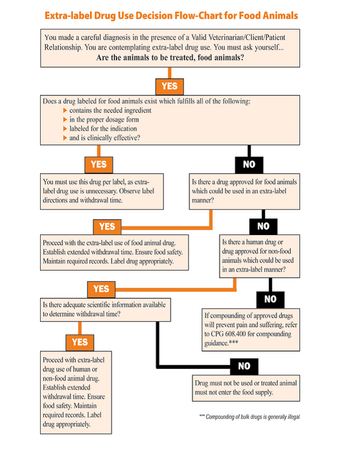 Extra Label Drug Use Flow Chart Elsakanner Wiki Fandom
Extra Label Drug Use Flow Chart Elsakanner Wiki Fandom
Extra Label Use Of Pharmaceutical Products Vet360
 Overview Of Laws Regulating Antibiotics In Livestock Amp Policy Positio
Overview Of Laws Regulating Antibiotics In Livestock Amp Policy Positio
 Dairy Moos Letter Medford Veterinary Clinic
Dairy Moos Letter Medford Veterinary Clinic
 Veterinary Pharmacology And The Veterinary Technician Ppt Download
Veterinary Pharmacology And The Veterinary Technician Ppt Download
The Fda And Animal Drug Compounding Neal Bataller Dvm Fda
 Palm Beach Veterinary Association March 9 Ppt Download
Palm Beach Veterinary Association March 9 Ppt Download
 Diagnosing And Treating Diseases
Diagnosing And Treating Diseases
 Pdf Veterinary Drug Residues In Food Animal Products Its Risk
Pdf Veterinary Drug Residues In Food Animal Products Its Risk
Wildlife Farad S Species Pages
 Pdf Health Canada S Policy On Extra Label Drug Use In Food
Pdf Health Canada S Policy On Extra Label Drug Use In Food
 Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
 Enrofloxacin Flavored Putney Inc Veterinary Package Insert
Enrofloxacin Flavored Putney Inc Veterinary Package Insert
 New Animal Drugs Orders Of Prohibition Cephalosporin Drugs
New Animal Drugs Orders Of Prohibition Cephalosporin Drugs
 Resources The Following Resources May Aid Practitioners In Keeping
Resources The Following Resources May Aid Practitioners In Keeping
 Application Of Risk Assessment And Management Principles To The
Application Of Risk Assessment And Management Principles To The
 Pdf Extralabel Use Of Ivermectin And Moxidectin In Food Animals
Pdf Extralabel Use Of Ivermectin And Moxidectin In Food Animals
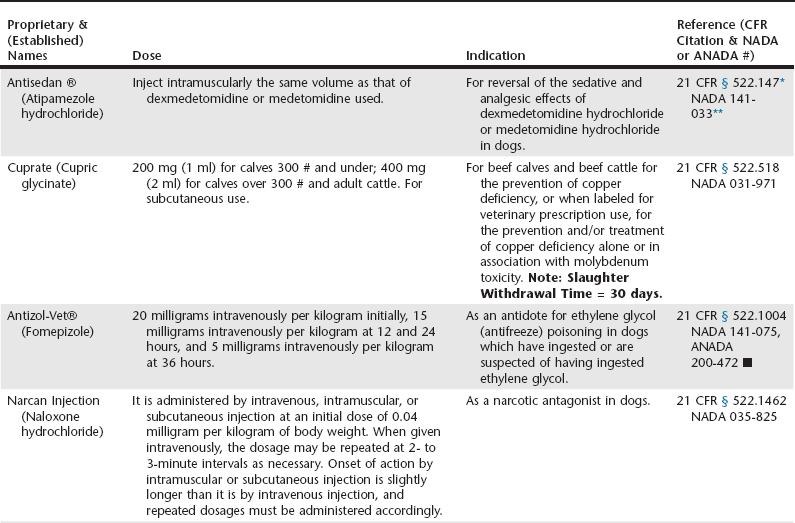 Chapter 10 Regulatory Points To Consider Veterian Key
Chapter 10 Regulatory Points To Consider Veterian Key
 Education Continuing Education Veterinary Forum Notes Nc
Education Continuing Education Veterinary Forum Notes Nc
 Medications Used In Small Ruminants And Camelids
Medications Used In Small Ruminants And Camelids
 Milk And Dairy Beef Drug Residue Prevention Pdf Free Download
Milk And Dairy Beef Drug Residue Prevention Pdf Free Download
 Table 6 From Drug Laws And Regulations For Sheep And Goats
Table 6 From Drug Laws And Regulations For Sheep And Goats
 Extralabel Use Of Tranquilizers And General Anesthetics Farad
Extralabel Use Of Tranquilizers And General Anesthetics Farad
 Pdf Tips And Tricks For Compounding Medications For Avian Patients
Pdf Tips And Tricks For Compounding Medications For Avian Patients
 Certification Training Ppt Download
Certification Training Ppt Download
 Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
Drug Use Guide Goats Lionel J Dawson Bvsc Ms Diplomate Act
 Wild Amp Woolly Winter 2018 By Md Small Ruminant Program Issuu
Wild Amp Woolly Winter 2018 By Md Small Ruminant Program Issuu
 Vcpr S And Prescription Drug Use Battenkill Veterinary Bovine
Vcpr S And Prescription Drug Use Battenkill Veterinary Bovine


Post a Comment for "34 Extra Label Drug Use"