33 Which Identifier Is Used To Mark The Label Of A Controlled Substance_
Identify brand and generic names on drug labels. Which identifier is used to mark the label of a controlled substance.
 Understanding Shipping Labels And Placards For Radioactive
Understanding Shipping Labels And Placards For Radioactive
Supplier labels and workplace labels.
.png?upscale=True)
Which identifier is used to mark the label of a controlled substance_. By using a dea approved disposal company. There are two main types of whmis labels. 2 it states the amount of the controlled substance to be.
In this final column of a 4 part series on key components of the federal controlled substances act dispensing requirements electronic controlled substances prescriptions and fraudulent controlled substances prescriptions will be discussed. What is the best way to dispose of an expired controlled substance. What does it mean to dispense a.
If the hazardous product is always used in the container with the supplier label no other label is required. A controlled substance is generally a drug or chemical whose manufacture possession or use is regulated by a government such as illicitly used drugs or prescription medications that are designated by law. What does the term controlled substance mean.
Ems frequently asked questions updated 6132019. The same symbol as above with the words very toxic is used to label a substance which if inhaled or ingested or if it penetrates the skin may involve extremely serious immediate or long term health risks and even death. Identify dosage strengths of medications on drug labels.
Positive identification means a method of identifying ems personnel that does not rely solely on the use of a private personal identifier such as a password but must include a secure means of identification such. The pharmacist dispensing a prescription for a controlled substance listed in schedules ii iii iv or v must affix to the package a label showing date of filling the pharmacy name and address the serial prescription number the name of the patient the name of the prescribing practitioner and directions for use and cautionary statements. Suppliers of hazardous products are required to apply a label that meets the requirements of the hazardous products regulations.
Learn vocabulary terms and more with flashcards games and other study tools. Start studying drug safety unit. All commercial containers of a controlled substance must be labeled with identification sysmbols designating the schedule in which the drug has been placed.
General prescription requirements for controlled substances. Immediate administration of controlled substance is medically necessary for the proper treatment of the intended ultimate user. Some treaties notably the single convention on narcotic drugs the convention on psychotropic substances and the united nations convention against illicit traffic in narcotic drugs and.
A a written prescription or an oral prescription reduced to writing when issued for a controlled substance in schedule ii iii iv or v is void unless 1 it is written in ink and contains the name and address of the person for whose use it is intended. Terms brand name chemical name controlled substance generic name innovator name national drug code proprietary name reconstitution trade name unit dose objectives upon completion of this chapter the technician student will be able to. Mark not accepted by supplier o the 2nd order form if.
 Understanding Electronic Prescribing
Understanding Electronic Prescribing
.png?upscale=True) Job Opportunities County Of San Benito
Job Opportunities County Of San Benito
 Abstract 2017 Pharmacoepidemiology And Drug Safety Wiley
Abstract 2017 Pharmacoepidemiology And Drug Safety Wiley
 Prescription Drugs Minnesota Prevention Resource Center
Prescription Drugs Minnesota Prevention Resource Center
 Improper Drug Dosage Wrong Medication Interactions With Other
Improper Drug Dosage Wrong Medication Interactions With Other
 Case And Pallet Packaging Under A Serialization Mandate
Case And Pallet Packaging Under A Serialization Mandate
 Pdf How E Labels Can Support Trade And Innovation In Ict
Pdf How E Labels Can Support Trade And Innovation In Ict
 Placebo Controlled Crossover Assessment Of Mecasermin For The
Placebo Controlled Crossover Assessment Of Mecasermin For The
Safety And Immunogenicity Of A Herpes Zoster Subunit Vaccine In
Prescription Labels And Drug Safety Consumer Reports
 Clinical Trial Design And Dissemination Comprehensive Analysis Of
Clinical Trial Design And Dissemination Comprehensive Analysis Of
Trademark Failure To Function Iowa Law Review The University
 Iuid Faqs Iuid Frequently Asked Questions Mil Std 130
Iuid Faqs Iuid Frequently Asked Questions Mil Std 130
 Glossary Of Shipping Terms Download Incodocs
Glossary Of Shipping Terms Download Incodocs
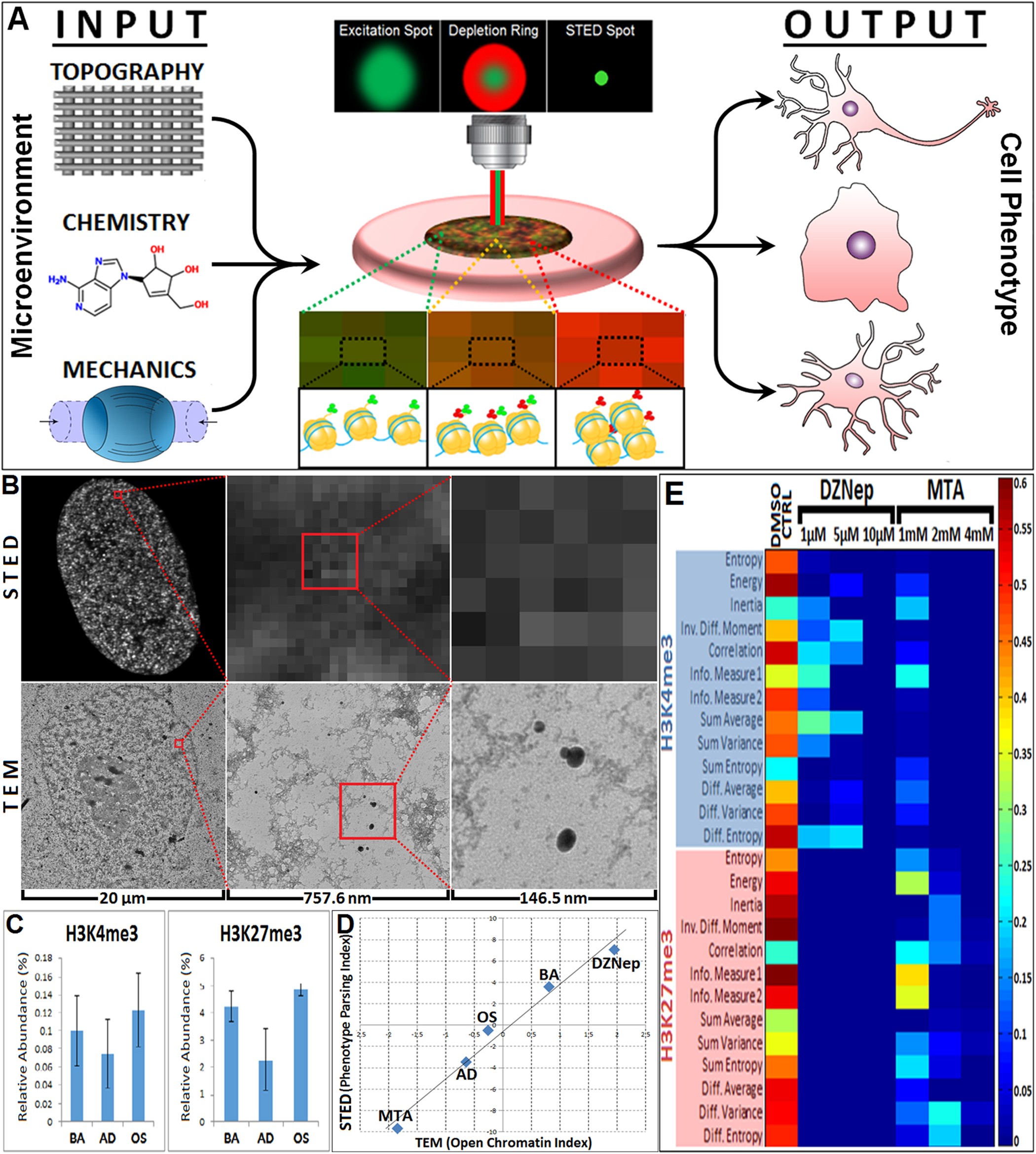 Optical High Content Nanoscopy Of Epigenetic Marks Decodes
Optical High Content Nanoscopy Of Epigenetic Marks Decodes
 Understanding Electronic Prescribing
Understanding Electronic Prescribing
 Consumer Effects Of Front Of Package Nutrition Labeling An
Consumer Effects Of Front Of Package Nutrition Labeling An
 Risk Of Death Following Application Of Paclitaxel Coated Balloons
Risk Of Death Following Application Of Paclitaxel Coated Balloons
 Aspen Safe Practices For Enteral Nutrition Therapy Boullata
Aspen Safe Practices For Enteral Nutrition Therapy Boullata
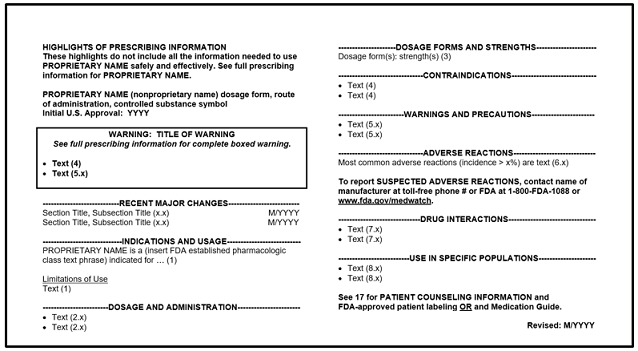 Prescription Drug Labeling Resources Fda
Prescription Drug Labeling Resources Fda
 Fighting Drug Diversion In Outpatient Settings Pharmacy Practice
Fighting Drug Diversion In Outpatient Settings Pharmacy Practice
 Over The Counter Drug Wikipedia
Over The Counter Drug Wikipedia
 Gate Ac Uk Sale Tao Index Html
Gate Ac Uk Sale Tao Index Html
Category Accounting California Cannabis Cpa
 Country Of Origin Labeling For Foods And The Wto Trade Dispute On
Country Of Origin Labeling For Foods And The Wto Trade Dispute On
 Pdf Openvigil Fda Inspection Of U S American Adverse Drug
Pdf Openvigil Fda Inspection Of U S American Adverse Drug
 Restrictions On The Use Of Prescribing Data For Drug Promotion Nejm
Restrictions On The Use Of Prescribing Data For Drug Promotion Nejm
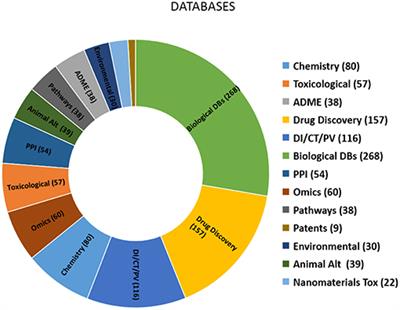 Frontiers In Silico Toxicology Data Resources To Support Read
Frontiers In Silico Toxicology Data Resources To Support Read
Trademark Failure To Function Iowa Law Review The University
 Pdf Comment On Deep Learning For Pharmacovigilance Recurrent
Pdf Comment On Deep Learning For Pharmacovigilance Recurrent
 Gpd1 Specifically Marks Dormant Glioma Stem Cells With A Distinct
Gpd1 Specifically Marks Dormant Glioma Stem Cells With A Distinct

Post a Comment for "33 Which Identifier Is Used To Mark The Label Of A Controlled Substance_"