30 Open Label Study Bias
Patients may research the new drug and its sideeffects in publications and may be influenced in their reporting behaviour of potential sideeffects. Naturally in openlabel trials in anticoagulation there is a risk of a reporting bias of adverse events.
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
The control treatment was prazosin alone.

Open label study bias. Detection and reporting bias. Open label studies lack the rigor of blinded studies. This is the approach used in trigger an open label cluster randomised trial in patients with acute upper gastrointestinal bleeding.
Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting recovery from scorpion sting. The primary clinical outcome was further bleeding. Liberal in patients admitted to uk hospitals with acute upper gastrointestinal bleeding augib.
In these situations it may be useful to modify the outcome definition to remove the most subjective elements thereby reducing the risk of bias. Blinded outcome assessment is recommended in open label trials to reduce bias however it is not always feasible. Randomised controlled trial open label unmasked subjective outcome unblinded outcome assessment bias background the main goal of a randomised controlled trial rct is to ensure that apart from the intervention there are no systematic differences between treatment groups under study thereby ensuring an unbiased estimate of treatment effect.
To minimise the risk of bias related to the open label nature of the trial the outcome measures were objective and to avoid the potential for treatment contamination individual practices were. In particular both the researchers and participants know which treatment is being administered. For example trigger transfusion in gastrointestinal bleeding was an open label cluster randomized trial that assessed the feasibility of implementing two red blood cell transfusion strategies restrictive vs.
An open label trial or open trial is a type of clinical trial in which information is not withheld from trial participants. The setting was a hospital and research centre in mahad a region of india. It is therefore important to find other means of reducing bias in these scenarios.
This contrasts with a blinded experiment where information is withheld to reduce bias. We describe two randomised trials where blinded outcome assessment was not possible and discuss the strategies used to reduce the possibility of bias. An open label randomised controlled trial study design was used.
The primary clinical outcome was an episode of further bleeding arising from the patients upper gastrointestinal tract. Since the lack of blinding can introduce significant bias reserve the use of open label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective such as survival.
 Risk Of Bias Across Included Studies Download Table
Risk Of Bias Across Included Studies Download Table
 Increased Risk Of Adverse Neurocognitive Outcomes With Proprotein
Increased Risk Of Adverse Neurocognitive Outcomes With Proprotein
Long Term Efficacy And Safety Of Evolocumab In Patients With
 Effects Of Placebos Without Deception Compared With No Treatment
Effects Of Placebos Without Deception Compared With No Treatment
 Endovascular Treatment Versus Standard Medical Treatment For
Endovascular Treatment Versus Standard Medical Treatment For
 4 Ways You Might Display Hidden Bias Every Day Cnn
4 Ways You Might Display Hidden Bias Every Day Cnn
 Study Characteristics Of Five Open Label Single Arm Intervention
Study Characteristics Of Five Open Label Single Arm Intervention
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
 Follow Up Interviews From The Salford Lung Study Copd And
Follow Up Interviews From The Salford Lung Study Copd And
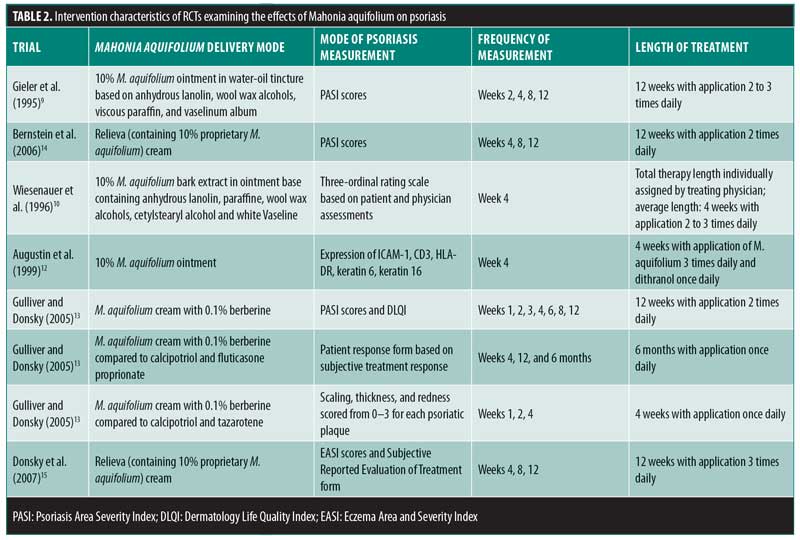 Review Of The Efficacy And Safety Of Topical Mahonia Aquifolium
Review Of The Efficacy And Safety Of Topical Mahonia Aquifolium
 Epidemiology And Clinical Research Design Part 1 Study Types
Epidemiology And Clinical Research Design Part 1 Study Types
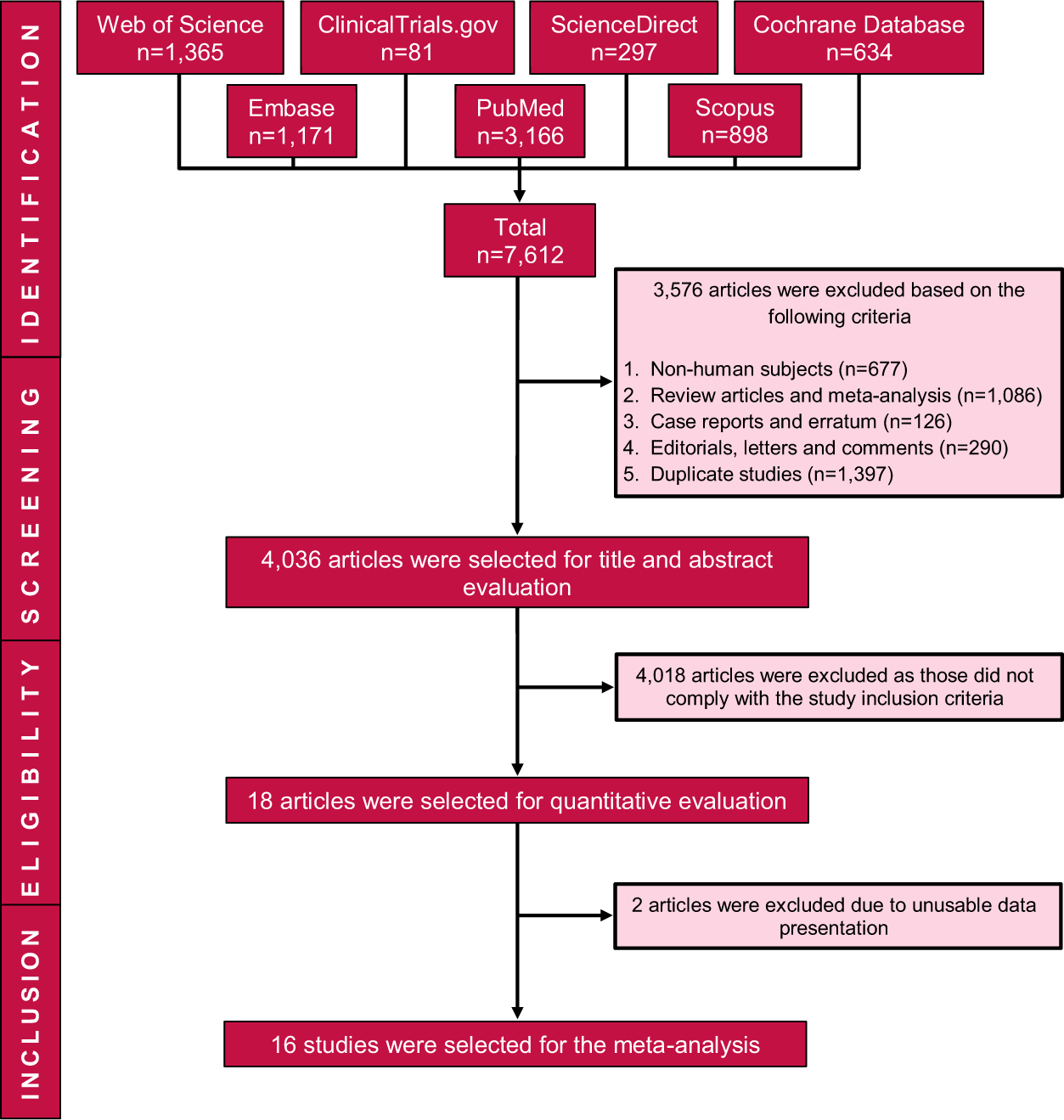 Efficacy And Safety Of Pioglitazone Monotherapy In Type 2 Diabetes
Efficacy And Safety Of Pioglitazone Monotherapy In Type 2 Diabetes
 Figure 1 From An Open Label Study On The Supplementation Of
Figure 1 From An Open Label Study On The Supplementation Of
 Media Bias Amp Criticism Definition Types Amp Examples
Media Bias Amp Criticism Definition Types Amp Examples
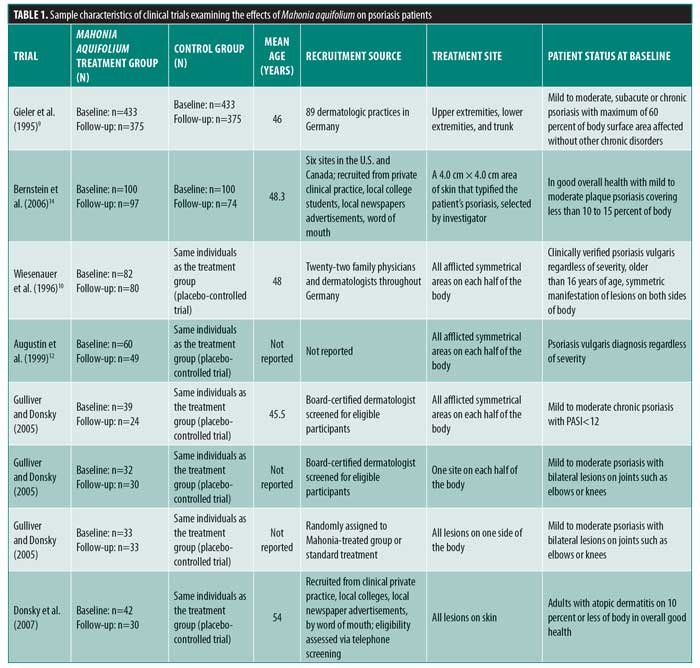 Review Of The Efficacy And Safety Of Topical Mahonia Aquifolium
Review Of The Efficacy And Safety Of Topical Mahonia Aquifolium
 Best Intentions Won T Solve Implicit Bias In Health Care Tmc News
Best Intentions Won T Solve Implicit Bias In Health Care Tmc News
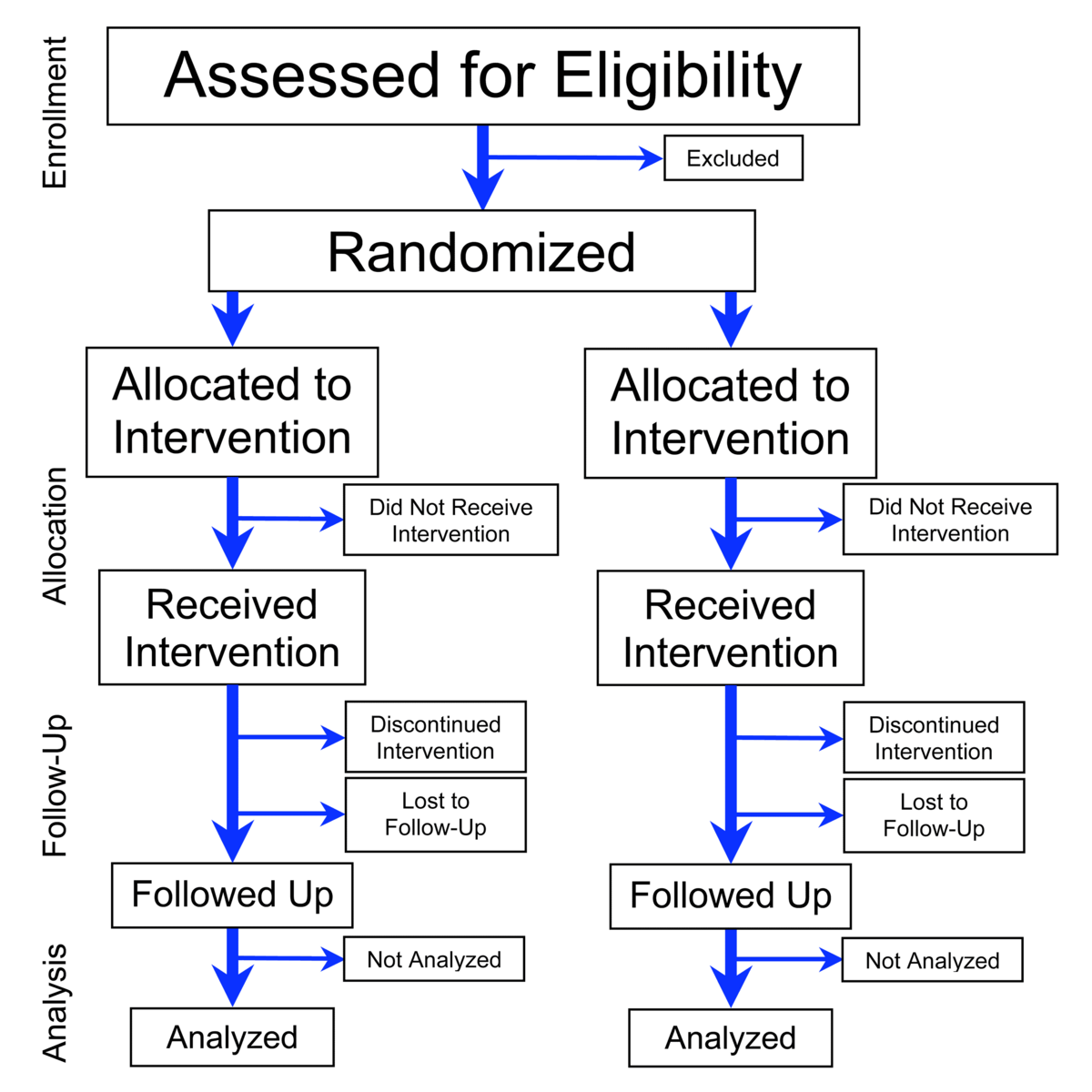 Randomized Controlled Trial Wikipedia
Randomized Controlled Trial Wikipedia
 Cherish Clinical Trial Efficacy Actemra Tocilizumab
Cherish Clinical Trial Efficacy Actemra Tocilizumab
 Design Characteristics Risk Of Bias And Reporting Of Randomised
Design Characteristics Risk Of Bias And Reporting Of Randomised
 Study Level Assessment Of Bias According To The Cochrane Risk Of
Study Level Assessment Of Bias According To The Cochrane Risk Of
 Phase 4 Postmarketing Research Springerlink
Phase 4 Postmarketing Research Springerlink
 Zanamivir For Influenza In Adults And Children Systematic Review
Zanamivir For Influenza In Adults And Children Systematic Review
 Design Characteristics Risk Of Bias And Reporting Of Randomised
Design Characteristics Risk Of Bias And Reporting Of Randomised
 The Tamiflu Debacle Rebel Em Emergency Medicine Blog
The Tamiflu Debacle Rebel Em Emergency Medicine Blog
Comparative Effectiveness Of Generic And Brand Name Medication Use
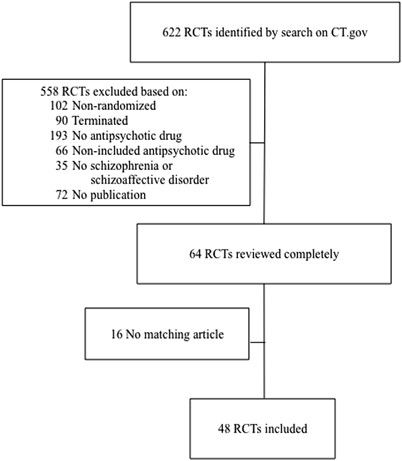 Outcome Reporting Bias In Randomized Controlled Trials
Outcome Reporting Bias In Randomized Controlled Trials
 Study Designs Randomization Bias Errors Power P Value Sample Size
Study Designs Randomization Bias Errors Power P Value Sample Size

Post a Comment for "30 Open Label Study Bias"