34 Off Label Promotion Medical Device
Allegations often include violations of the federal food drug and cosmetic act the false claims act and the anti kickback statute. The food and drug administration fda has statutory authority to regulate the advertising and promotion of restricted medical devices as well as any medical devices that are not authorized by fda for sale or distribution for their intended use.
 Fda Is Illegally Allowing The Off Label Promotion Of Medical
Fda Is Illegally Allowing The Off Label Promotion Of Medical
And benefit information for prescription drugs and medical devices.

Off label promotion medical device. Fda guidance documents regarding advertising and promotion. To know more about the promotion advertising rules for medical devices contact us. Heres how fda officials think you can legally promote off label device drug uses june 13 2018 by nancy crotti the fda has issued final guidance clarifying what it considers permissible off label information for device makers and drug companies to convey to potential customers.
As a summary any medical device promotional or samples of medical devices to be made available on the eu market must be in conformity with the above regulatory frameworks either on the device the outer packaging or the ifus. The company allegedly promoted lc bead its embolization devicedesigned to be inserted into blood vessels to block the flow of blood to tumorsfor off label use as a drug delivery device which was not an fda approved use and was not supported by substantial clinical evidence. Implicit off label promotion occurs when a manufacturer indirectly suggests that a device is appropriate for an unapproved use.
The fda has previously developed draft guidance responding to unsolicited requests for off label information about prescription drugs and medical devices that addresses how manufacturers can respond to unsolicited requests for off label information relating to their fda approved or cleared products without such responses being used as evidence of intended use. Promoting off label use of a medical device a use not included on a devices indication with fda has become a dangerous practice for medtech insiders with some facing potential prison time for alleged off label advertising. Off label investigational use of marketed drugs biologics and medical devices information sheet.
For example in 2000 a medical device company that manufactured a product cleared for use as an adjunctive diagnostic screening device for detecting breast cancer received a warning letter from fda. It is conducted in compliance with the requirements concerning the promotion and sale of. Requests for off label information about.
There are tens of thousands of medical devices being advertised and promoted in the us. For medical device the consequences of promoting a product for off label use could be significant.
 Regulating Off Label Drug Use Rethinking The Role Of The Fda Nejm
Regulating Off Label Drug Use Rethinking The Role Of The Fda Nejm
/cdn.vox-cdn.com/uploads/chorus_image/image/63003808/sponcon3.0.png) Instagram Influencers Are Selling You Drugs And Medical Devices Vox
Instagram Influencers Are Selling You Drugs And Medical Devices Vox
 Medtronic To Pay 2 8 Million To Settle Off Label Promotion
Medtronic To Pay 2 8 Million To Settle Off Label Promotion
 Fda Regulation Of The Dissemination Of Medical Device Information
Fda Regulation Of The Dissemination Of Medical Device Information
 Tips To Avoid Off Label Promotion
Tips To Avoid Off Label Promotion
Off Label Promotion For Medical Devices Pdf Free Download
American Health Lawyers Journal October 2018 Page 102
 Generic Medical Device Company Mdc Ppt Powerpoint
Generic Medical Device Company Mdc Ppt Powerpoint
 Updated Off Label Promotion Are Fda S Rules About To Unravel Raps
Updated Off Label Promotion Are Fda S Rules About To Unravel Raps
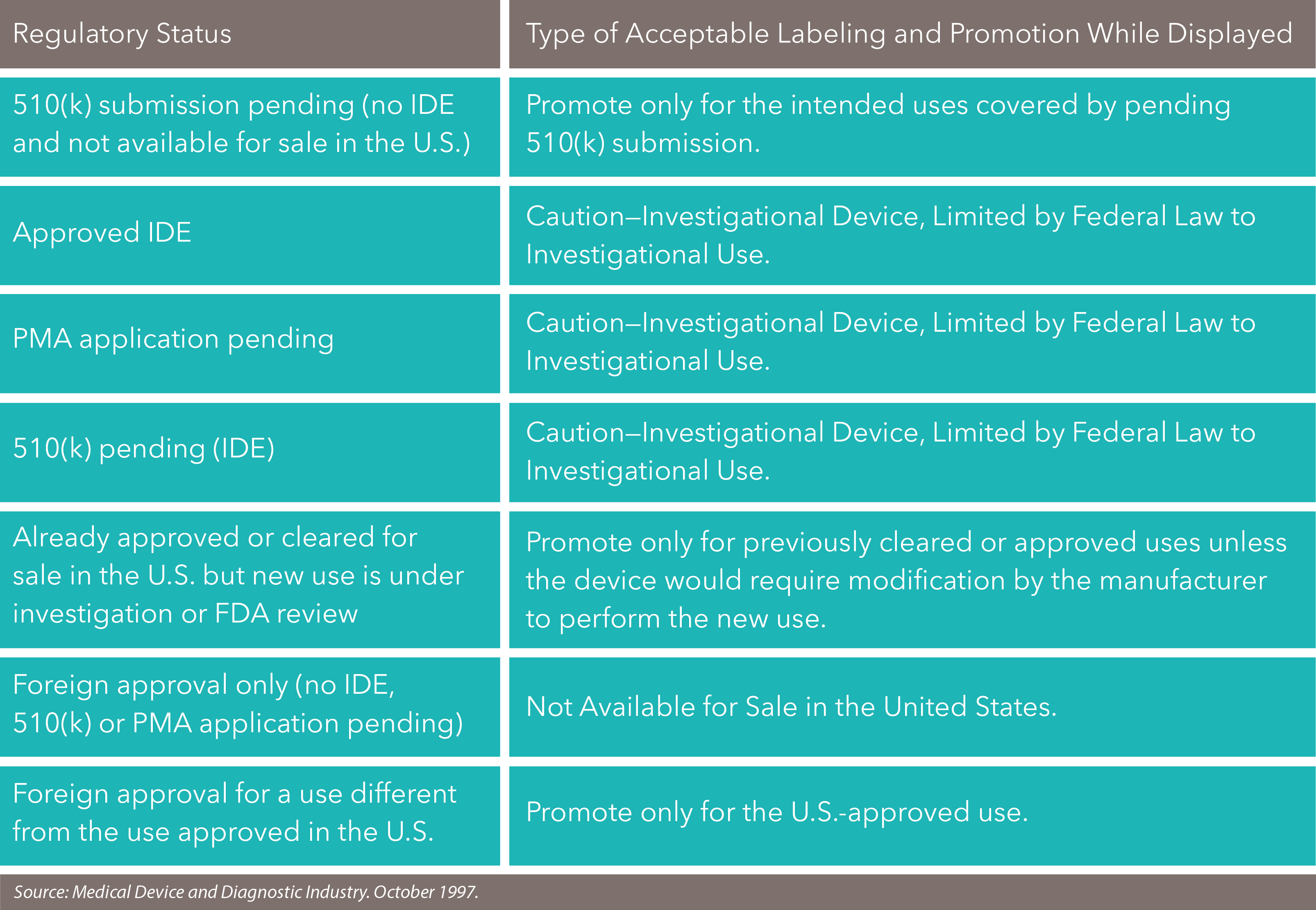 Promoting Medical Devices Prior To Fda Approval Or Clearance
Promoting Medical Devices Prior To Fda Approval Or Clearance
 Here S How Fda Officials Think You Can Legally Promote Off Label
Here S How Fda Officials Think You Can Legally Promote Off Label
 Off Label Promotion Is Not Going Away Darshan Kulkarni
Off Label Promotion Is Not Going Away Darshan Kulkarni
Off Label Promotion For Medical Devices Pdf Free Download
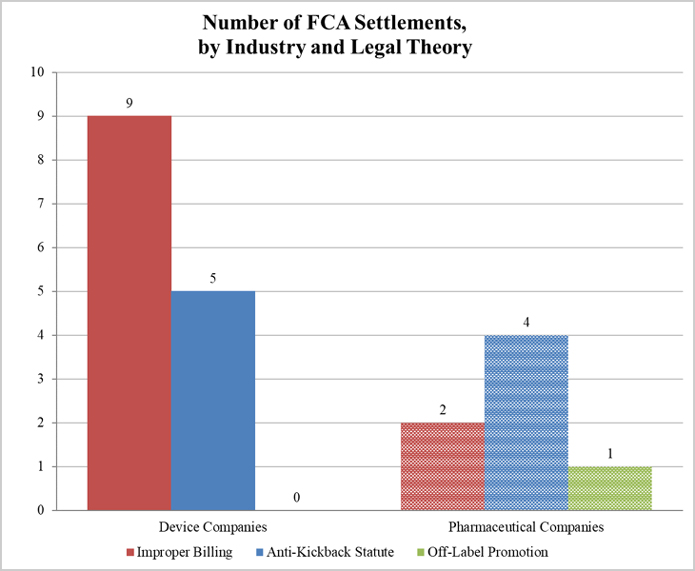 Gibson Dunn 2018 Year End Fda And Health Care Compliance And
Gibson Dunn 2018 Year End Fda And Health Care Compliance And
 Fda Issues Final Rule On Symbols In Medical Device Labeling
Fda Issues Final Rule On Symbols In Medical Device Labeling
Pharmaceutical Industry Off Label Promotion And Self Regulation A
 Fda Ropes Amp Gray Ropes Amp Gray Llp
Fda Ropes Amp Gray Ropes Amp Gray Llp
 News Fda Claims No Change In Off Label Guidance Policy
News Fda Claims No Change In Off Label Guidance Policy
 Fda Is Illegally Allowing The Off Label Promotion Of Medical
Fda Is Illegally Allowing The Off Label Promotion Of Medical
 10 Causes Of Medical Device Failure Mddi Online
10 Causes Of Medical Device Failure Mddi Online
 Fda Is Illegally Allowing The Off Label Promotion Of Medical
Fda Is Illegally Allowing The Off Label Promotion Of Medical
 Us And Eu Veterinary Medical Device Regulation Raps
Us And Eu Veterinary Medical Device Regulation Raps
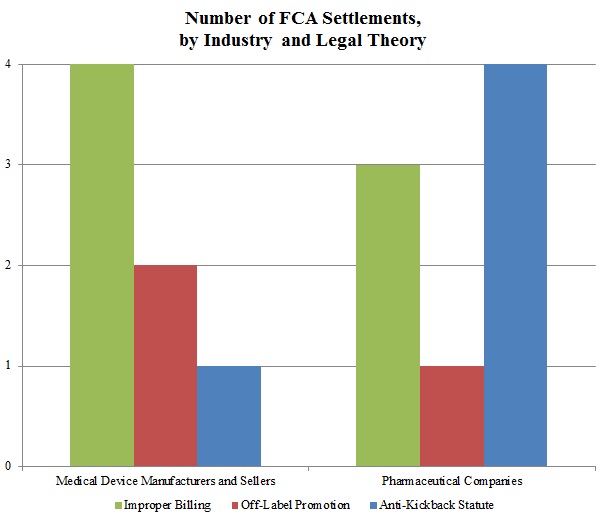 Gibson Dunn 2015 Year End Fda And Health Care Compliance And
Gibson Dunn 2015 Year End Fda And Health Care Compliance And
 Tips To Avoid Off Label Promotion
Tips To Avoid Off Label Promotion
 Fda Allows Off Label Health Care Economic Discussions Is More To
Fda Allows Off Label Health Care Economic Discussions Is More To
Pharmaceutical Industry Off Label Promotion And Self Regulation A
 Fda Guidance Responding To Unsolicited Request For Off Label
Fda Guidance Responding To Unsolicited Request For Off Label
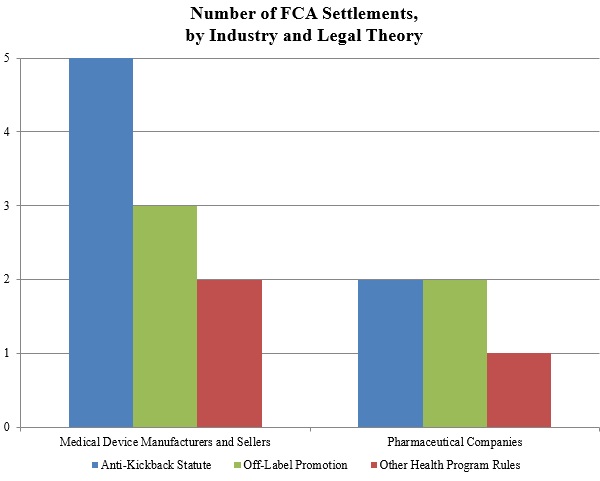 Gibson Dunn 2016 Year End Fda And Health Care Compliance And
Gibson Dunn 2016 Year End Fda And Health Care Compliance And
 Drug Minnow Amarin Downs Fda In Constitutional Battle
Drug Minnow Amarin Downs Fda In Constitutional Battle
 Fda Regulation Of Medical Device Advertising And Promotion
Fda Regulation Of Medical Device Advertising And Promotion
 The Regulatory Authority For Off Label Promotion Ppt Download
The Regulatory Authority For Off Label Promotion Ppt Download
Advertising Medicaldeviceslegal

Post a Comment for "34 Off Label Promotion Medical Device"