34 What Is Off Label Prescribing
What is off label prescribing. Food and drug administration fda has approved to treat a condition different than your condition.
 Why Doctors Prescribe Off Label Drugs Scientific American
Why Doctors Prescribe Off Label Drugs Scientific American
The fda requires that drugs go through a clinical testing process to establish that theyre safe and effective to treat a particular condition.
What is off label prescribing. Off label means the medication is being used in a manner not specified in the fdas approved packaging label or insert. Carries an individual fda approved label. This term can mean that the drug is.
Every prescription drug marketed in the us. This is a dangerous practice that is common among doctors and tolerated by the fda. Off label prescribing is when a physician gives you a drug that the us.
This practice is legal and common. In fact one in five prescriptions written today are for off label use. Used for a disease or medical condition that it is not approved to treat such as when a.
Its also considered off label prescribing if the drug is given to a patient to treat a condition it was not approved to treat. What exactly is off label prescribing. Off label use means that the medicine is being used in a way that is different to that described in the licence.
This label is a written report that provides detailed instructions regarding. Unapproved use of an approved drug is often called off label use. Use of a prescription drug in higher doses is known as off label prescribing.
Healthcare providers generally may prescribe the drug for an unapproved use when they judge that it is medically appropriate for their patient.
 Study More Doctors Are Prescribing Off Label Medications To
Study More Doctors Are Prescribing Off Label Medications To
 Pdf Off Label Prescribing In Older Patients
Pdf Off Label Prescribing In Older Patients
 Startups Are Hawking Zoloft And Beta Blockers For Off Label Uses
Startups Are Hawking Zoloft And Beta Blockers For Off Label Uses
 Off Label Prescribing Of Antipsychotics For Youths Who Should Be
Off Label Prescribing Of Antipsychotics For Youths Who Should Be
 Taking Flak For Off Label Prescribing Multiple Sclerosis
Taking Flak For Off Label Prescribing Multiple Sclerosis
 Concerns Regarding The Safety And Efficacy Of Off Label
Concerns Regarding The Safety And Efficacy Of Off Label
 Patients Experiment With Prescription Drugs To Fight Aging
Patients Experiment With Prescription Drugs To Fight Aging
 Off Label Prescribing Justifying Unapproved Medicine
Off Label Prescribing Justifying Unapproved Medicine
 Off Label Prescribing Is Legitimate But You Should Ask A Few
Off Label Prescribing Is Legitimate But You Should Ask A Few
 Off Label Prescription Drugs What You Need To Know
Off Label Prescription Drugs What You Need To Know
 Medminute Doctors Prescribing More Off Label Medications For Kids
Medminute Doctors Prescribing More Off Label Medications For Kids
 Off Label Pediatric Medicine Why Pediatricians Prescribe Adult
Off Label Pediatric Medicine Why Pediatricians Prescribe Adult
 When Is Off Label Prescribing Appropriate Mdedge Psychiatry
When Is Off Label Prescribing Appropriate Mdedge Psychiatry
 Eu Ruling Likely To Set An Increase In Off Label Use Of Medicinal
Eu Ruling Likely To Set An Increase In Off Label Use Of Medicinal

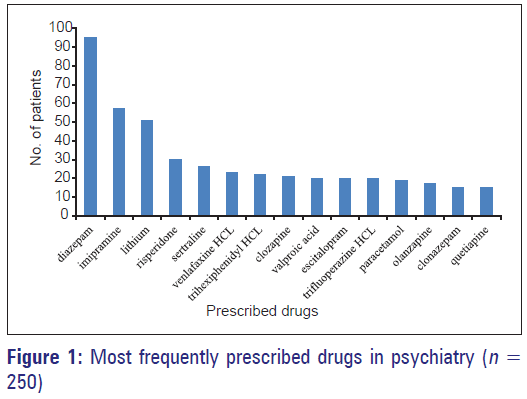 Off Label Drug Use In Psychiatry Outpatient Department A
Off Label Drug Use In Psychiatry Outpatient Department A
Pharmaceutical Industry Off Label Promotion And Self Regulation A
.jpg) Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
 Do S And Don Ts For Off Label Prescribing
Do S And Don Ts For Off Label Prescribing
The Common Yet Unspoken Practice Of Off Label Drug Prescribing
 Factors Associated With Off Label Prescribing In Univariate
Factors Associated With Off Label Prescribing In Univariate
 It May Be Legal But Is It Ethical To Advertise Prescription Drugs
It May Be Legal But Is It Ethical To Advertise Prescription Drugs
 Lakartidningen Off Label Prescribing Of Hormones In Gender
Lakartidningen Off Label Prescribing Of Hormones In Gender
Off Label Drugs Require More Study News Center Stanford Medicine

Pharmaceutical Industry Off Label Promotion And Self Regulation A
Patterns And Predictors Of Off Label Prescription Of Psychiatric Drugs




Post a Comment for "34 What Is Off Label Prescribing"