31 What Is Off Label
Off label means the medication is being used in a manner not specified in the fdas approved packaging label or insert. An exception to this is the use of some controlled substances such as opioids pain medicines like morphine and fentanyl.
 When Should Insurers Cover Off Label Drug Usage Managed Care
When Should Insurers Cover Off Label Drug Usage Managed Care
How to use off label in a sentence.

What is off label. Both prescription drugs and over the counter drugs otcs can be used in off label ways although most studies of off label use focus on prescription drugs. This label is a written report that provides detailed instructions regarding. Is off label drug use legal.
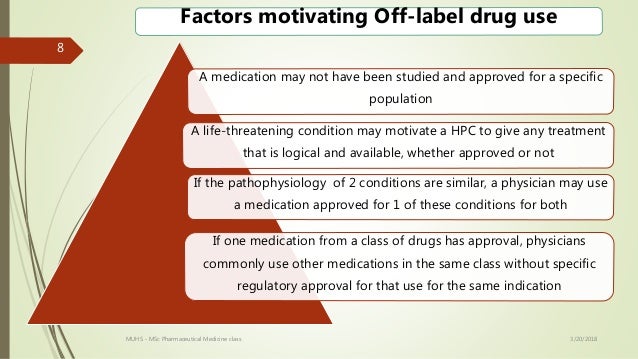
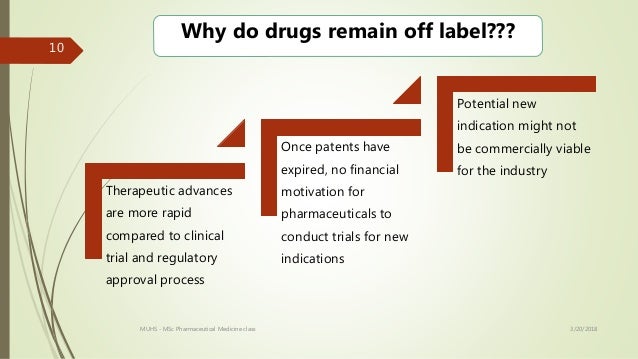
In fact one in five prescriptions written today are for off label use. When the fda approves a drug the approved labeling specifies among other things the diseases or conditions for which the drug has been approved by the fda. Off label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group dosage or route of administration.
Has your healthcare provider ever talked to you about using an fda approved drug for an unapproved use sometimes called an off label use to treat your disease or medical condition. The off label use of fda approved drugs is not regulated but it is legal in the united states and many other countries. Off label prescribing is when a physician gives you a drug that the us.
Every prescription drug marketed in the us. Off label definition is of relating to or being a drug used to treat a condition for which it has not been officially approved. The term off label drug use sounds pretty serious like it might be risky or illegal but its actually very common.
When a doctor prescribes medication for off label use this means that the physician is prescribing a drug in a way that is different than what is in the fda approved labeling for the drug. Food and drug administration fda has approved to treat a condition different than your condition. Still if you read about prescription drug lawsuits or scandals in the media it can seem like off label use always hurts patients or leads to litigation.
This practice is legal and common. Of relating to or being a drug used to treat a condition for which it has not been officially approved see the full definition. Off label is also called non approved or unapproved use of a drug.
Carries an individual fda approved label.
The Common Yet Unspoken Practice Of Off Label Drug Prescribing
Researcher Criticizes Plan To Reduce Off Label Drug Oversight
 Set 1 Of Cute And Colorful Peeling Off Label Or Sticker In Primary
Set 1 Of Cute And Colorful Peeling Off Label Or Sticker In Primary
Off Label Use Of Non Opioids For Pain Appear To Rise In Response
 Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
 The Fda S Approach To Off Label Communications Restricting Free
The Fda S Approach To Off Label Communications Restricting Free
 Off Label Drug Use Why Do Doctors Prescribe Them
Off Label Drug Use Why Do Doctors Prescribe Them
 Off Label Use Of Antidepressants For Chronic Pain Clinical Pain
Off Label Use Of Antidepressants For Chronic Pain Clinical Pain
 Off Label Drug Use In Wound Care Woundsource
Off Label Drug Use In Wound Care Woundsource
 Intranasal Medications In Clinical Practice Ppt Video Online
Intranasal Medications In Clinical Practice Ppt Video Online
 Fda Sanctions Off Label Drug Promotion Health Affairs
Fda Sanctions Off Label Drug Promotion Health Affairs
.jpg) Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
 Explainer Why Are Off Label Medicines Prescribed
Explainer Why Are Off Label Medicines Prescribed
 Astrazeneca Agrees To Settle Off Label Marketing Suits Policy
Astrazeneca Agrees To Settle Off Label Marketing Suits Policy
 A Personal Health Advisor Educates On Prescription Drugs
A Personal Health Advisor Educates On Prescription Drugs
 Amarin V Fda And Public Meeting On Off Label Promotion Signal An
Amarin V Fda And Public Meeting On Off Label Promotion Signal An
 Use Of Off Label Medications In The Treatment Of Chronic Insomnia
Use Of Off Label Medications In The Treatment Of Chronic Insomnia
 Why Doctors Prescribe Off Label Drugs Scientific American
Why Doctors Prescribe Off Label Drugs Scientific American
 As Off Label Use Spreads Supplies Of Niche Drugs And Patients
As Off Label Use Spreads Supplies Of Niche Drugs And Patients
 Off Label Prescribing Is Legitimate But You Should Ask A Few
Off Label Prescribing Is Legitimate But You Should Ask A Few
 Medicare Denials Of Off Label Drug Use Medicare Made Clear
Medicare Denials Of Off Label Drug Use Medicare Made Clear
 Special Offer 30 Off Label Or Price Tag Stock Vector
Special Offer 30 Off Label Or Price Tag Stock Vector
 Off Label Uses Of Octreotide Download Table
Off Label Uses Of Octreotide Download Table
 Off Label Uses For Treating Pain
Off Label Uses For Treating Pain



Post a Comment for "31 What Is Off Label"