30 Open Label Study Design
Open label trials may be performed to study the effectiveness of similar medications. Study design and provide a rationale for the proposed study design.
Spotlight On Open Label Extension Studies Applied Clinical Trials
Open label extension ole studies are common but they do not receive as much attention as traditional phase i through phase iv studies.
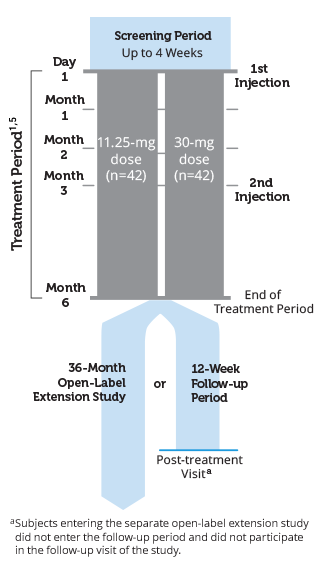
Open label study design. Discusses design options for enrichment strategies and discusses the interpretation of the results of studies that use enrichment strategies. Open label extension studies typically follow a double blind randomised placebo controlled trial of a new drug. Participants are usually informed at the time they are recruited into the main study that they may elect to enroll in an ole study after.
Enrollment into an ole study typically follows enrollment into a randomized blinded well controlled main study. Open label study is a type of clinical trial in which the researchers and participants or parents know which treatment is being administered. For non masked open label studies this means before any patient reaches an outcome.
This contrasts with single blind and double blind designs. Because this was an open label trial the participants investigators and all peripheral staff were not blinded to the treatment allocationthat is they were aware which treatment the participants had been allocated to after. Participants in a clinical trial are typically provided a great deal of information about the trials design.
Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects 6 10. This contrasts with a blinded experiment where information is withheld to reduce bias. The control treatment was prazosin alone.
Open label extension studies. Many trial designs do not permit blinding and are therefore designed as open label with patients clinicians and other study investigators aware of treatment allocation. For instance while dabigatran was tested in af using an open label study 1 and in acute deep vein thrombosis dvt and pulmonary embolism pe using a double blind double dummy trial 2 rivaroxaban was tested inversely with open label trials in acute dvt and pe 3.
In particular both the researchers and participants know which treatment is being administered. An open label study may still be randomized. Double blind double dummy table 1.
Newly developed medications can be compared with their predecessors in an open label trial. To the trial design of open label vs. At the end of the double blind phase participants are invited to enrol in an extension study.
In general fdas guidance documents do not. An open label trial or open trial is a type of clinical trial in which information is not withheld from trial participants. An open label randomised controlled trial study design was used.
Gtl001 A Therapeutic Vaccine For Women Infected With Human
 Study Design Elzonris Tagraxofusp Erzs
Study Design Elzonris Tagraxofusp Erzs
 Diagram Showing The Study Design For The 12week Open Label Study
Diagram Showing The Study Design For The 12week Open Label Study
 Design Of A Phase Iv Randomised Double Blind Placebo Controlled
Design Of A Phase Iv Randomised Double Blind Placebo Controlled
 Stelara Ustekinumab Efficacy Crohn S Disease
Stelara Ustekinumab Efficacy Crohn S Disease
 Study Design Pfizer For Professionals
Study Design Pfizer For Professionals
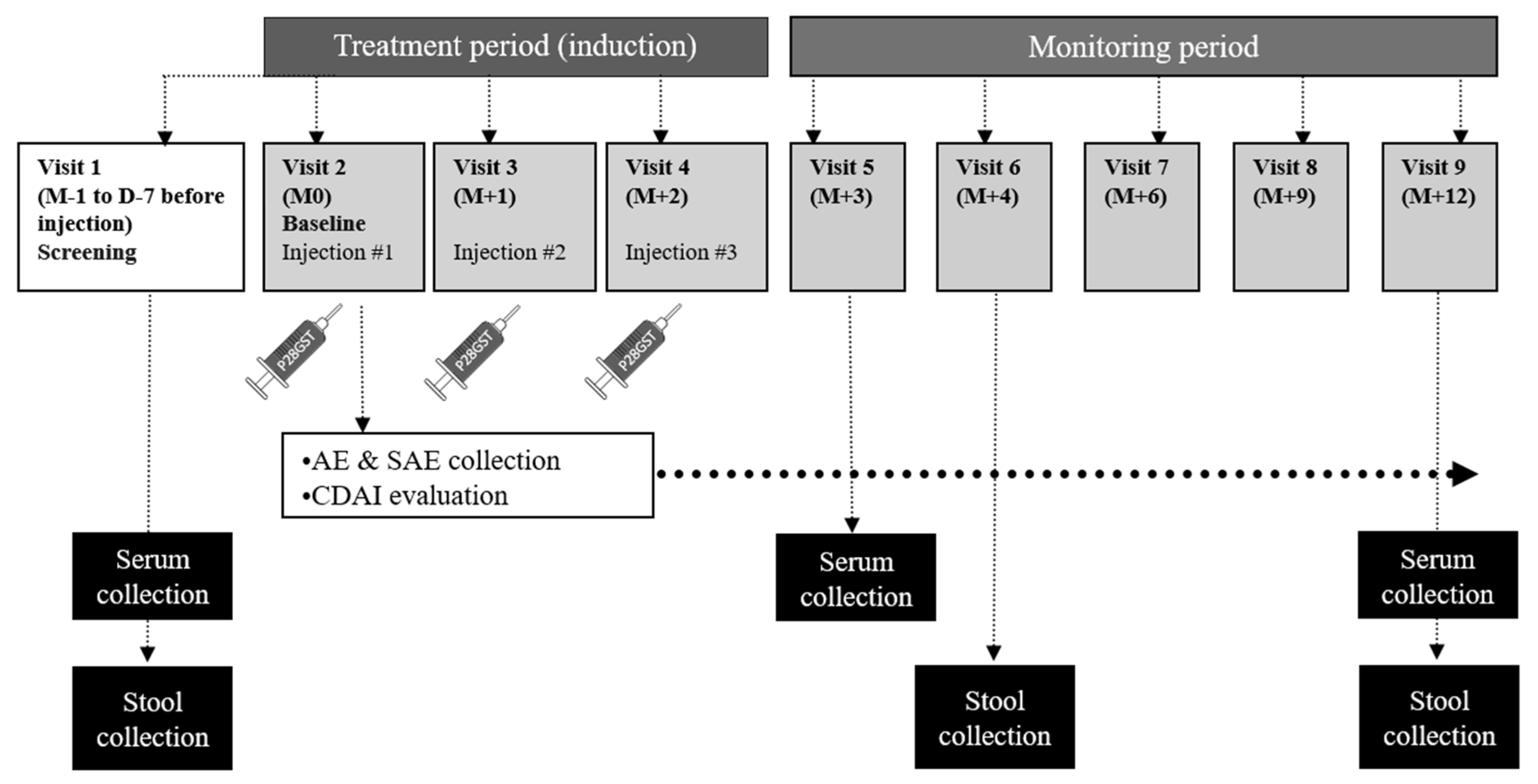 Jcm Free Full Text Safety Of P28gst A Protein Derived From A
Jcm Free Full Text Safety Of P28gst A Protein Derived From A

 Hanan Goldberg On Twitter Dr De Wit Presenting Keynote 057
Hanan Goldberg On Twitter Dr De Wit Presenting Keynote 057
 Study Design Of Clinical Trial Open Label Multiple Dose
Study Design Of Clinical Trial Open Label Multiple Dose
 Most Women Use Vaginal Ring For Hiv Prevention In Open Label Study
Most Women Use Vaginal Ring For Hiv Prevention In Open Label Study

 A Phase 2 Randomized Dose Finding Study Of The Novel Once Weekly
A Phase 2 Randomized Dose Finding Study Of The Novel Once Weekly
Rigel Announces Results From The Second Fit Phase 3 Study And The
 Clinical Studies Lupron Depot Ped Leuprolide Acetate For Depot
Clinical Studies Lupron Depot Ped Leuprolide Acetate For Depot

 Cardiovascular Clinical Trials In Japan And Controversies
Cardiovascular Clinical Trials In Japan And Controversies
 Protocol For A Randomised Open Label Parallel Group Multicentre
Protocol For A Randomised Open Label Parallel Group Multicentre
Rationale And Design Of A Multi Center Open Label Randomised
 Study Design Pfizer For Professionals
Study Design Pfizer For Professionals
 Evenity Vs Placebo Study Design Evenity Hcp Site
Evenity Vs Placebo Study Design Evenity Hcp Site
Imerge Study Myelodysplastic Syndromes Clinical Trial Imetelstat
 Evenity Vs Teriparatide Study Design Evenity Hcp Site
Evenity Vs Teriparatide Study Design Evenity Hcp Site
 Study Design For Pivotal Phase Iii Trial Cs21 And Its Open Label
Study Design For Pivotal Phase Iii Trial Cs21 And Its Open Label
 Study Design For Pioneer I And Ii And Open Label Extension
Study Design For Pioneer I And Ii And Open Label Extension
 Open Label Switch Study Latuda Lurasidone Hcl For Sz
Open Label Switch Study Latuda Lurasidone Hcl For Sz
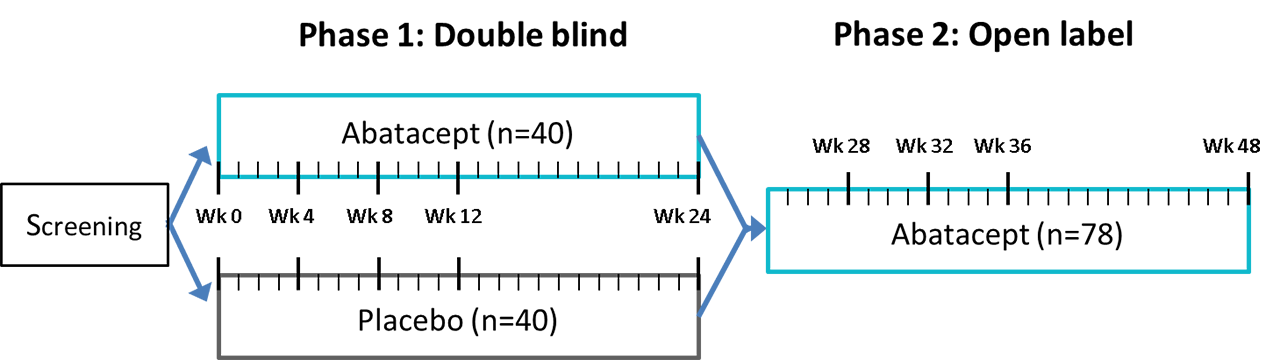 Efficacy And Safety Of Abatacept In Patients With Early Active
Efficacy And Safety Of Abatacept In Patients With Early Active
Metabolic Evaluation Of Study M05 730 Through Week Wk 48 Phase

Post a Comment for "30 Open Label Study Design"