32 Off Label Use Fda
Unapproved use of an approved drug is often called off label use. Off label drug use is when drugs are prescribed for a condition or a type of patient or a dosage not officially approved by the us.
 Drug Minnow Amarin Downs Fda In Constitutional Battle
Drug Minnow Amarin Downs Fda In Constitutional Battle
Food and drug administration.

Off label use fda. The term off label drug use oldu is used extensively in the medical literature continuing medical education exercises and the media. Yet we propose that many health care professionals have an underappreciation of its definition prevalence and implications. The agency has no control over how doctors prescribed approve drugs to patients.
The off label use of fda approved drugs is not regulated but it is legal in the united states and many other countries. At a different dose or frequency. In the past large drug manufacturers have been fined by the justice department for intentionally marketing their drugs for off label use exposing patients and doctors to undue risk.
Used for a disease or medical condition that it is not approved to treat such as when a chemotherapy is approved to treat one type of cancer but healthcare providers use it to treat a different type of cancer. Off label use of a drug or combination of drugs often represents the standard of care. This term can mean that the drug is.
Off label and investigational use of marketed drugs biologics and medical devices guidance for institutional review boards and clinical investigators january 1998. According to the american cancer society cancer treatment often involves using certain chemotherapy drugs off label because a chemotherapy drug approved for one type of cancer may actually target many different types of tumors. If a manufacturer could promote off label uses without a supplemental application cortez purports that the manufacturer would not take on the expense of a supplemental application.
The fdca requires substantial evidence to approve a new drug but most off label uses lack the substantial evidence necessary for fda approval. The fda does not regulate the medical profession. Off label uses may include using an approved drug.
To treat a child when it is approved to treat adults. For a different type of cancer than the one it is approved to treat. An exception to this is the use of some controlled substances such as opioids pain medicines like morphine and fentanyl.
Problematic off label drug use in most cases off label drug use provides more benefit than risk but it does occasionally cause problems. Fda would subsequently not be able to guarantee that there was substantial evidence that every drug on the market was safe and effective. Off label uses of a drug can become approved uses if the company that makes it obtains approval from the fda.
 Off Label Drug Use And Fda Review Of Supplemental Drug Applications Hearing Before The Subcommittee On Human Resources And Intergovernmental
Off Label Drug Use And Fda Review Of Supplemental Drug Applications Hearing Before The Subcommittee On Human Resources And Intergovernmental
Medications Widely Prescribed For Unproven Treatments
 Article Off Label Medication And Use Of Unapproved Drugs Citation Lu
Article Off Label Medication And Use Of Unapproved Drugs Citation Lu
 Off Label Use Of Atypical Antipsychotics An Update Comparative
Off Label Use Of Atypical Antipsychotics An Update Comparative
 F D A Deal Allows Amarin To Promote Drug For Off Label Use The
F D A Deal Allows Amarin To Promote Drug For Off Label Use The
 The State Of Off Label Drug Laws Dermatology Times
The State Of Off Label Drug Laws Dermatology Times
 Will More Data To Be Available On Off Label Drug Uses 2017 10
Will More Data To Be Available On Off Label Drug Uses 2017 10
 Fda Off Label May Be The Wrong Label Policy Amp Medicine
Fda Off Label May Be The Wrong Label Policy Amp Medicine
 Fda Warns Against Off Label Use Of Stryker S Wingspan Stent
Fda Warns Against Off Label Use Of Stryker S Wingspan Stent
 Fda Amarin Agree Company Can Promote Off Label Use Of
Fda Amarin Agree Company Can Promote Off Label Use Of
 Off Label Drug Marketing To Be Reviewed During Fda Meeting
Off Label Drug Marketing To Be Reviewed During Fda Meeting
 Regulating Off Label Drug Use Rethinking The Role Of The Fda Nejm
Regulating Off Label Drug Use Rethinking The Role Of The Fda Nejm
 Here S How Fda Officials Think You Can Legally Promote Off Label
Here S How Fda Officials Think You Can Legally Promote Off Label
 Fda Cautions Against The Off Label Use Of Surgical Robotics Mddi
Fda Cautions Against The Off Label Use Of Surgical Robotics Mddi
 Distribution Of Off Label Antidepressants And Fda Approved
Distribution Of Off Label Antidepressants And Fda Approved
 Racial Ethnic Differences In Pediatric Antipsychotic Use By Fda
Racial Ethnic Differences In Pediatric Antipsychotic Use By Fda
 Under Pressure Fda To Hold Public Meeting On Off Label Use
Under Pressure Fda To Hold Public Meeting On Off Label Use
 Overview Of Fda Regulation Of Medical Devices
Overview Of Fda Regulation Of Medical Devices
 Off Label Non Fda Approved Indications For Topical Imiquimod
Off Label Non Fda Approved Indications For Topical Imiquimod
 Life Underwriting Expert Series Off Label Drug Use
Life Underwriting Expert Series Off Label Drug Use
 Facetime With Phil Fda S New Position On Off Label Drug Use
Facetime With Phil Fda S New Position On Off Label Drug Use
 Amarin V Fda And Public Meeting On Off Label Promotion Signal An
Amarin V Fda And Public Meeting On Off Label Promotion Signal An
 Off Label Use Of Drugs C3ihc Drug Safety Amp Healthcare Outsourcing
Off Label Use Of Drugs C3ihc Drug Safety Amp Healthcare Outsourcing
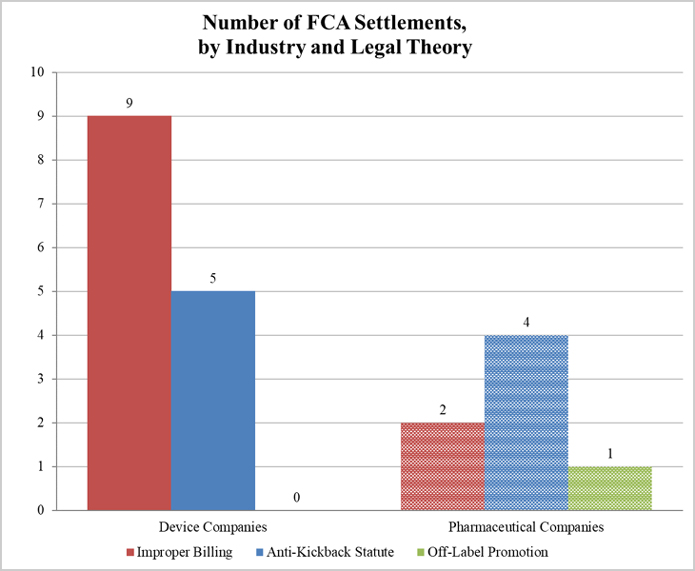 Gibson Dunn 2018 Year End Fda And Health Care Compliance And
Gibson Dunn 2018 Year End Fda And Health Care Compliance And
Off Label Drug Use In Oncology Accounts For 18 Of Spending
Fda Approved Medications Used For The Treatment Of Prostate Cancer
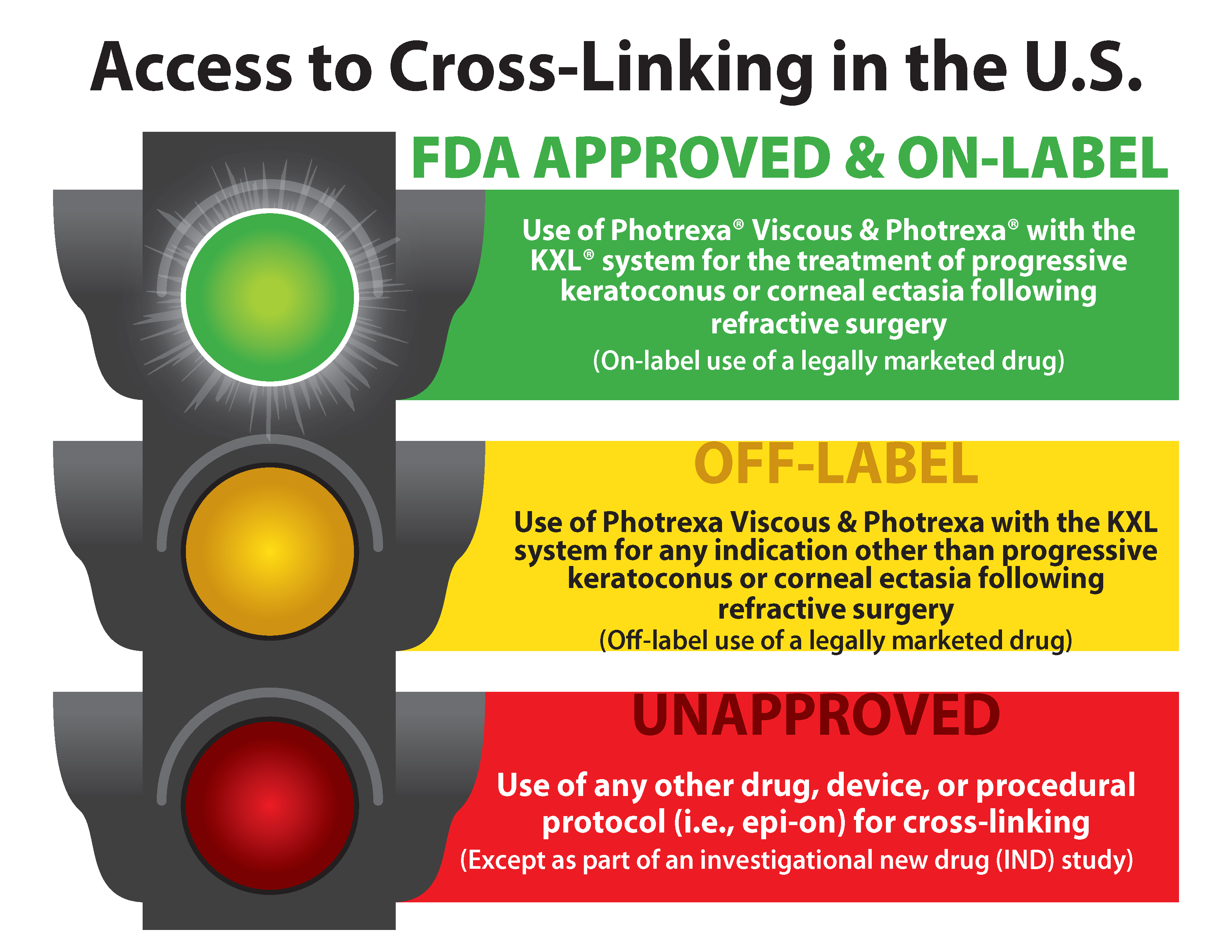 What To Know About Fda Approved Treatments Living With Keratoconus
What To Know About Fda Approved Treatments Living With Keratoconus
%2C445%2C291%2C400%2C400%2Carial%2C12%2C4%2C0%2C0%2C5_SCLZZZZZZZ_.jpg) The Guide To Off Label Prescription Drugs New Uses For Fda
The Guide To Off Label Prescription Drugs New Uses For Fda
 Fda Warns Of Respiratory Problems Linked To Off Label Tramadol Use
Fda Warns Of Respiratory Problems Linked To Off Label Tramadol Use
American Health Lawyers Journal February 2019 Page 1
Post a Comment for "32 Off Label Use Fda"