30 Medical Device Label Requirements
The mdr will become enforceable in 2020 and introduces new rules relating to labeling requirements for medical devices. This includes the physical address of the manufacturer.
 Label Examples Hct P Medical Devices Iccbba
Label Examples Hct P Medical Devices Iccbba
Labelling for medical devices.

Medical device label requirements. Device advice introduction to labeling requirements for medical devices including advertising over the counter exemptions in vitro diagnostics investigational devices quality system. 131 each device must be accompanied by the information needed to use it safely and properly taking account of the training and knowledge of the potential users 231a the medium format content legibility and location of the label and instructions for use shall be appropriate to the particular device its intended. For medical devices article 133 of directive 9342eec annex 1 essential requirements sets out what if applicable must be stated on the label.
Labelling serves to communicate safety and performance related information to users of medical devices andor patients as well as to identify individual devices. Most people think that medical device labeling is just the label on the device or box and dont think about the ifu. When we talk about labeling were talking about the labels on the pouch on the box etc and the instructions for use ifu.
Important considerations for labelling of medical devices under the new eu mdr in european market regulatory by dr. On may 5 2017 the european commission released the new europe eu medical device regulations mdr in an effort to create a more unified and transparent system for medical devices. Introduction to medical device labeling label vs.
Every medical device and ivd must be provided with a label. The regulatory requirements of some countries may not at present reflect the contents of this document. Susanne beckert january 25 2017 the new european medical device regulation mdr gives great importance to the information supplied by a manufacturer to the end user to assist with the safe and proper use of a medical device.
All medical devices and their accessories that have been placed on the european union eu market since june 15 1998 have had to comply with the essential requirements set forth by the medical devices directive including the ce mark and labeling requirements. According to the fda labeling is. Medical device labeling is not just the label on your device.
The essential requirements of the medical device directive 9342eec referred to as the mdd set out the detailed requirements for labelling of medical devices in annex 1 paragraphs 87 132 and 133 a n. Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that.
Does Your Medical Device Have A Unique Device Identification Udi
The Fda S Udi Compliance Mandate In Plain English The Label
Compliance Route Determination And Ce Marking 510 K Strategy
 Medical Device Receiving Facilities Iccbba
Medical Device Receiving Facilities Iccbba
Fda Medical Device Labeling Regulations Archives Medical Device
 Never Use Whiteout Fda Proposes Change To Device Labels Symbols
Never Use Whiteout Fda Proposes Change To Device Labels Symbols
 Label Compliance And The New European Medical Device Regulations
Label Compliance And The New European Medical Device Regulations
Need For Harmonization Of Labeling Of Medical Devices A Review
 Destination Labeling Print On Demand Innovatum
Destination Labeling Print On Demand Innovatum
Medical Device Labels Manufacturing Market Industry Analysis
Mdmv6b Vimove Wireless Medical Device User Manual Dorsavi Pty
Medical Device And Ivd Registration In China
 Udi And The Impact On Medical Devices Part 2 Labeling Zuhlke
Udi And The Impact On Medical Devices Part 2 Labeling Zuhlke
 Medical Device Color Label Printers Paladinid Llc
Medical Device Color Label Printers Paladinid Llc
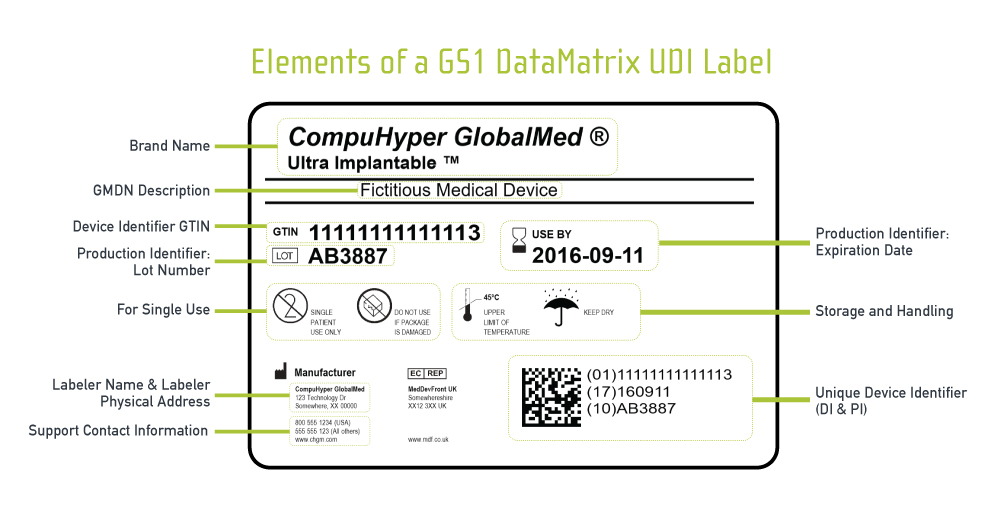 Udi Labeling Pitfalls And Best Practices Part 1 Of 2 Reed Tech
Udi Labeling Pitfalls And Best Practices Part 1 Of 2 Reed Tech
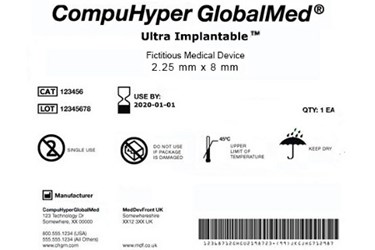 Fda S New Udi Rule What Device Makers Need To Know
Fda S New Udi Rule What Device Makers Need To Know
 Unique Device Identification Udi Stryker
Unique Device Identification Udi Stryker
Plos One Evaluating Varied Label Designs For Use With Medical
 International Labeling Requirements For Medical Devices Medical
International Labeling Requirements For Medical Devices Medical
 Advanced Label Worx Medical Device Labels From The Experts
Advanced Label Worx Medical Device Labels From The Experts
 Unique Device Identification Olympus Medical Systems
Unique Device Identification Olympus Medical Systems
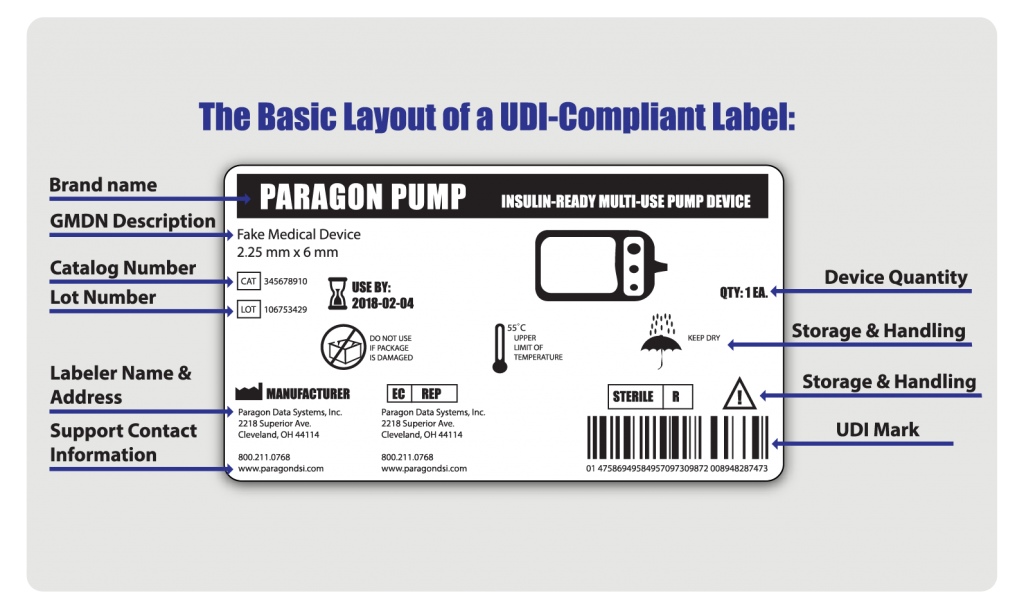 Udi Unique Device Identification For Single And Multiple Uses
Udi Unique Device Identification For Single And Multiple Uses
 Webinar Eu Mdr S Impact On Labeling Udi In Europe And Other
Webinar Eu Mdr S Impact On Labeling Udi In Europe And Other
 Implementing Udi Labelling For Medical Devices Doranix
Implementing Udi Labelling For Medical Devices Doranix
 A Recap Of The Medical Device Packaging Amp Labeling Summit
A Recap Of The Medical Device Packaging Amp Labeling Summit
Effort To Improve Tracking Of Medical Devices Divides Industry
 Medical Device Labeling Imprint Enterprises
Medical Device Labeling Imprint Enterprises


Post a Comment for "30 Medical Device Label Requirements"